The question is –
Is SCl4F2 polar or non-polar?
Answer:
⇒ SCl4F2 could be polar or non-polar depending upon the arrangement of outer atoms around the central S-atom.
In an arrangement where the two F-atoms oppositely point to each other, SCl4F2 is a non-polar molecule.
Explanation:
SCl4F2 consists of a sulfur (S) atom at the center. It is surrounded by 4 chlorine (Cl) atoms and 2 fluorine (F) atoms.
There are a total of four S-Cl single covalent bonds and two S-F bonds.
Each S-Cl bond is polar as per an electronegativity difference of 0.58 units between the sulfur (E.N = 2.58) and chlorine (E.N = 3.16) atoms.
Conversely, both S-F bonds are strongly polar, having an electronegativity difference of 1.40 units between the sulfur and fluorine (E.N = 3.98) atoms, respectively.
Thus, all S-Cl and S-F bonds possess specific dipole moment values (symbol µ).
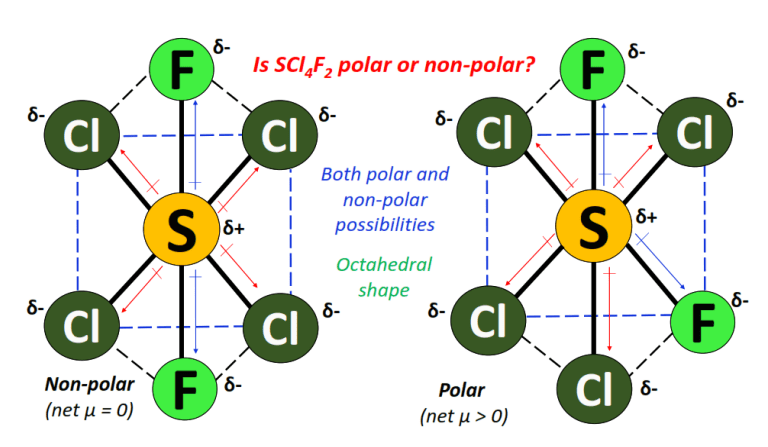
In the octahedral shape and molecular geometry of SCl4F2, if the bonded atoms are symmetrically arranged such that the 4 Cl-atoms occupy the four corners of the base while the 2 F-atoms lie on opposite sides, one on top and the other at the bottom of the base, then the S-Cl and S-F dipole moments get canceled equally.
This results in an overall non-polar molecule (net µ = 0).
In another possible arrangement, if the two F-atoms are not oppositely directed, the S-Cl and S-F dipole moments do not get canceled, then SCl4F2 is polar (net µ > 0).
⇒ How to know if a molecule is polar or nonpolar?

