The question is –
Is OH– polar or nonpolar?
Answer:
⇒ OH– is polar.
Explanation:
The hydroxide (OH–) ion is polar due to a high electronegativity difference between the covalently bonded oxygen and hydrogen atoms which leads to a strong dipole moment value.
As per Pauling’s electronegativity scale, a polar covalent bond is formed between two dissimilar atoms having an electronegativity difference ranging from 0.4 to 1.6 units.
The OH– ion consists of only a single covalent bond i.e., an O-H bond.
The O-H bond is strongly polar as an electronegativity difference of 1.24 units is present between an oxygen (E.N = 3.44) and a hydrogen (E.N = 2.20) atom.
Oxygen, being more electronegative than hydrogen, strongly attracts the O-H electron cloud towards itself.
It thus gains a partial negative charge (δ–), while the H-atom gains a partial positive charge (δ+), respectively.
In this way, oppositely charged poles develop in OH–.
The strong dipole moment of the O-H bond stays uncancelled in the linear shape of OH– ion.
The charged electron cloud stays unequally distributed over the hydroxide ion.
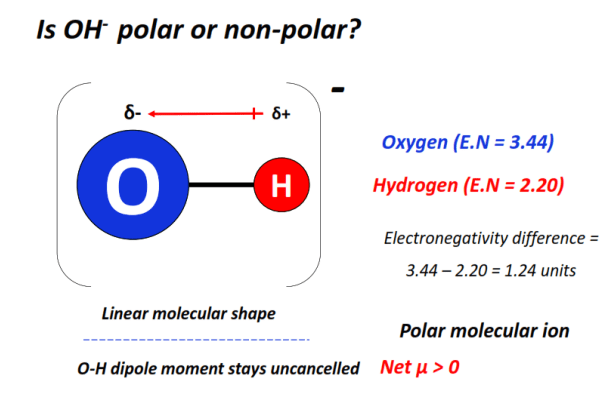
The presence of a -1 formal charge on the oxygen atom and, thus, on the molecular ion as a whole further enhances the polarity effect.
Consequently, OH– is overall polar (net µ > 0).
Also, check –
⇒ How to identify polar or nonpolar compounds?
⇒ Is OH– an acid or base?

