The question is –
Is MgCl2 polar or nonpolar?
Answer:
⇒ MgCl2 is polar.
Explanation:
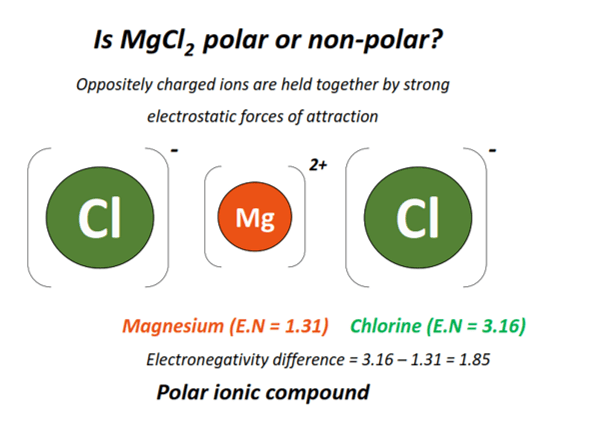
Magnesium chloride (MgCl2) is a polar ionic compound as it consists of oppositely charged ions held together by strong electrostatic forces of attraction.
All ionic compounds are polar in nature.
Each unit cell of MgCl2 consists of an Mg2+ ion and 2 Cl– ions.
As per Pauling’s electronegativity scale, a polar ionic compound is formed between two dissimilar atoms having an electronegativity difference of greater than 1.6 units.
Magnesium (Mg) is an electropositive element. It belongs to the alkaline Earth metals (Group II A of the Periodic Table), having an electronegativity value of 1.31.
In contrast, chlorine (Cl) is a halogen from Group VII A of the Periodic Table. It is highly electronegative, with an electronegativity value of 3.16.
Therefore, an electronegativity difference of 1.85 units exists between a magnesium and a chlorine atom.
Chlorine, being more electronegative, attracts the shared electron cloud largely toward itself.
It thus gains a permanent negative charge while magnesium attains a permanent positive charge.
Oppositely charged Mg2+ and 2 Cl– ions are held together by strong electrostatic forces of attraction, with a high dipole moment value to yield an extremely polar ionic compound (net µ > 0).
Like dissolves like; thus, the high solubility of MgCl2 in a polar solvent such as water (54.3 g anhydrous MgCl2 in 100 mL water) is another proof of the polar nature of magnesium chloride.
Also, check –
⇒ How to identify polar or nonpolar compounds?
⇒ Why MgCl2 is an ionic compound?

