Is food coloring polar or nonpolar? – (Polarity of food coloring)
The question is –
Is food coloring polar or nonpolar?
Answer:
⇒ Food coloring is most likely to be polar.
Explanation:
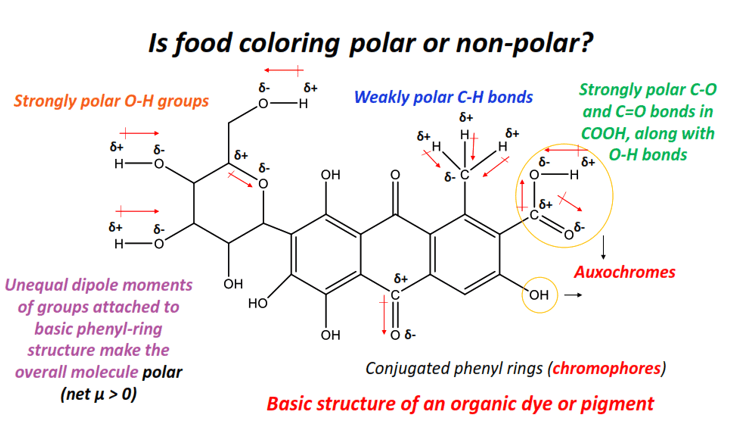
Food coloring is most likely to be polar as it is composed of dyes or pigments containing highly polar O-H, S=O, C-O, etc, bonds whose dipole moments stay uncancelled.
Organic dyes containing conjugated systems and one or more chromophores or auxochromes form the primary component of food colorings.
Conjugation refers to the interlinking of pi-bonded systems. If C-C single and C=C double covalent bonds are present at alternating positions, the pi-bonded electrons can move from one place to another.
Chromophores are functional groups that give a chemical compound its characteristic color by absorbing visible light (400-800) nm.
Auxochromes are functional groups that do not absorb visible light themselves, but when attached to a chromophore, it helps them in absorbing visible light of even a lower frequency (higher wavelength).
Conjugated phenyl rings are an example of chromophores, while functional groups such as O-H, COOH, C=O, -NH2, -SCH3, -SO32-, etc., are some prominent examples of auxochromes.
As per the above information, there are the following main types of covalent bonds present in food coloring:
- C-C or C=C
- C-H
- O-H
- C-O or C=O
- N-H
- S-O or S=O
A C-C or C=C is purely non-polar as zero, or no electronegativity difference exists between two identical carbon atoms.
A C-H bond is very weakly polar, having an electronegativity difference of 0.35 units between a carbon (E.N = 2.55) and a hydrogen (E.N = 2.20) atom.
An O-H bond is strongly polar as it consists of an electronegativity difference of 1.24 units between oxygen (E.N = 3.44) and hydrogen. So it possesses a high dipole moment value.
Similarly, C-O and N-H bonds are polar with electronegativity differences of 0.89 units and 0.84 units, respectively. An electronegativity difference of 0.86 units is present between bonded atoms in S-O or S=O bonds.
The strong dipole moments of all the above bonds stay uncancelled in the conjugated molecule i.e., an organic dye. Therefore, food colorings are overall polar (net µ > 0).
Also, check –
⇒ How to identify polar or nonpolar compounds?
Related Posts
- Is CH3CH2CH2OH polar or nonpolar? – Chemistry QnA
- Is OH2 polar or nonpolar? – Chemistry QnA
- Is ICl4- polar or nonpolar? – Chemistry QnA
- Is OH- polar or nonpolar? – Chemistry QnA
- Is SiS2 polar or nonpolar? – Chemistry QnA
- Is MgCl2 polar or nonpolar? – Chemistry QnA
- Is CaCl2 polar or nonpolar? – Chemistry QnA
- Is 2-propanol polar or nonpolar? – Chemistry QnA
- Are double bonds more polar than single bonds? – Chemistry QnA
- Is Octane (C8H18) polar or nonpolar? – Chemistry QnA
- Is S8 polar or nonpolar? – Chemistry QnA
- Is HCO3- polar or nonpolar? – Chemistry QnA
- Is SbF5 polar or nonpolar? – Chemistry QnA
- Is CH3CH2CH3 polar or nonpolar? – Chemistry QnA
- Is NH4Br polar or nonpolar? – Chemistry QnA
- Is HBrO polar or nonpolar? – Chemistry QnA
- Is SeCl2 polar or nonpolar? – Chemistry QnA
- Is IOF5 polar or nonpolar? – Chemistry QnA
- Is GeH4 polar or nonpolar? – Chemistry QnA
- Is CH4O polar or nonpolar? – Chemistry QnA
- Is KBr polar or nonpolar? – Chemistry QnA
- Is BrF polar or nonpolar? – Chemistry QnA
- Is C2H2Br2 polar or nonpolar? – Chemistry QnA
- Is SCl6 polar or nonpolar? – Chemistry QnA
- Is NO+ polar or nonpolar? – Chemistry QnA
- Is SeH2 polar or nonpolar? – Chemistry QnA
- Is Cl2O polar or nonpolar? – Chemistry QnA
- Is GaH3 polar or nonpolar? – Chemistry QnA
- Is KCl polar or nonpolar? – Chemistry QnA
- Is AlBr3 polar or nonpolar? – Chemistry QnA
- Is NaOH polar or nonpolar? – Chemistry QnA
- Is CH3CH3 polar or nonpolar? – Chemistry QnA
- Is Cyclohexane polar or nonpolar? – Chemistry QnA
- Is NH4NO3 polar or nonpolar? – Chemistry QnA
- Is SCl4F2 polar or nonpolar? – Chemistry QnA
- Is C2H6O polar or nonpolar? – Chemistry QnA
- Is HClO polar or nonpolar? – Chemistry QnA
- Is AsH3 polar or nonpolar? – Chemistry QnA
- Cl2XeF2 polar or nonpolar? – Chemistry QnA
- SeCl6 polar or nonpolar? – Chemistry QnA
About the author
Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. The team at Topblogtenz includes experts like experienced researchers, professors, and educators, with the goal of making complex subjects like chemistry accessible and understandable for all. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/
