Is CH3CH2CH2OH polar or nonpolar? – (Polarity of 1-propanol)
The question is –
Is 1-propanol (CH3CH2CH2OH) polar or nonpolar?
Answer:
⇒ 1-propanol (CH3CH2CH2OH) is polar.
Explanation:
1-propanol or propan-1-ol (CH3CH2CH2OH) is a polar primary alcohol as it consists of a strongly polar hydroxyl (OH) functional group in it.
The strong dipole moments of C-O and O-H bonds do not get canceled with the weak dipole moments of C-H bonds.
CH3CH2CH2OH is a primary alcohol as the hydroxyl (OH) functional group is present at the terminal position i.e., attached to the C-atom carrying one alkyl chain only.
It is composed of 1 O-H bond, 1 C-O bond, 2 C-C bonds, and 7 C-H bonds.
Each C-C bond is purely non-polar as it consists of two identical carbon atoms having zero or no electronegativity differences.
A C-H bond is very weakly polar as the covalently bonded carbon (E.N = 2.55), and hydrogen (E.N = 2.20) atoms have an electronegativity difference of only 0.35 units.
A C-O bond is strongly polar as an electronegativity difference of 0.89 units is present between a carbon and an oxygen (E.N = 3.44) atom.
The O-H bond is extremely polar, having an electronegativity difference of 1.24 units between the oxygen and hydrogen atoms.
The shape of CH3CH2CH2OH w.r.t each C-atom is tetrahedral while that w.r.t the O-atom is bent, angular, or V-shaped.
2 lone pairs of electrons present on the oxygen atom lead to strong lone pair-lone pair and lone pair-bond pair electronic repulsions, which in turn distorts the shape and geometry of the molecule.
Oxygen being strongly electronegative attracts electrons from each C-H bond in addition to attracting the O-H and C-O bonded electrons.
The O-atom thus gains a partial negative charge (δ–), while the C and H-atoms gain partial positive charges (δ+ and/or δ++) to denote an extreme electron deficiency.
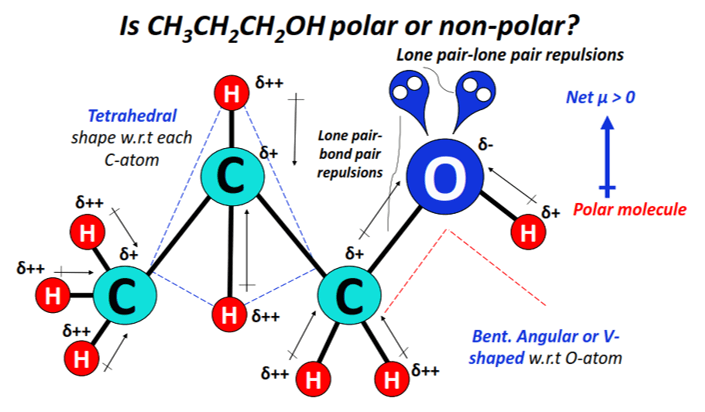
The strong O-H and C-O dipole moments do not get canceled equally.
The charged electron cloud stays non-uniformly distributed, which leads to an overall polar CH3CH2CH2OH molecule (net µ > 0).
Also, check –
⇒ How to identify polar or nonpolar compounds?
Related Posts
- Is OH2 polar or nonpolar? – Chemistry QnA
- Is ICl4- polar or nonpolar? – Chemistry QnA
- Is OH- polar or nonpolar? – Chemistry QnA
- Is SiS2 polar or nonpolar? – Chemistry QnA
- Is MgCl2 polar or nonpolar? – Chemistry QnA
- Is food coloring polar or nonpolar? – Chemistry QnA
- Is CaCl2 polar or nonpolar? – Chemistry QnA
- Is 2-propanol polar or nonpolar? – Chemistry QnA
- Are double bonds more polar than single bonds? – Chemistry QnA
- Is Octane (C8H18) polar or nonpolar? – Chemistry QnA
- Is S8 polar or nonpolar? – Chemistry QnA
- Is HCO3- polar or nonpolar? – Chemistry QnA
- Is SbF5 polar or nonpolar? – Chemistry QnA
- Is CH3CH2CH3 polar or nonpolar? – Chemistry QnA
- Is NH4Br polar or nonpolar? – Chemistry QnA
- Is HBrO polar or nonpolar? – Chemistry QnA
- Is SeCl2 polar or nonpolar? – Chemistry QnA
- Is IOF5 polar or nonpolar? – Chemistry QnA
- Is GeH4 polar or nonpolar? – Chemistry QnA
- Is CH4O polar or nonpolar? – Chemistry QnA
- Is KBr polar or nonpolar? – Chemistry QnA
- Is BrF polar or nonpolar? – Chemistry QnA
- Is C2H2Br2 polar or nonpolar? – Chemistry QnA
- Is SCl6 polar or nonpolar? – Chemistry QnA
- Is NO+ polar or nonpolar? – Chemistry QnA
- Is SeH2 polar or nonpolar? – Chemistry QnA
- Is Cl2O polar or nonpolar? – Chemistry QnA
- Is GaH3 polar or nonpolar? – Chemistry QnA
- Is KCl polar or nonpolar? – Chemistry QnA
- Is AlBr3 polar or nonpolar? – Chemistry QnA
- Is NaOH polar or nonpolar? – Chemistry QnA
- Is CH3CH3 polar or nonpolar? – Chemistry QnA
- Is Cyclohexane polar or nonpolar? – Chemistry QnA
- Is NH4NO3 polar or nonpolar? – Chemistry QnA
- Is SCl4F2 polar or nonpolar? – Chemistry QnA
- Is C2H6O polar or nonpolar? – Chemistry QnA
- Is HClO polar or nonpolar? – Chemistry QnA
- Is AsH3 polar or nonpolar? – Chemistry QnA
- Cl2XeF2 polar or nonpolar? – Chemistry QnA
- SeCl6 polar or nonpolar? – Chemistry QnA
About the author
Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. The team at Topblogtenz includes experts like experienced researchers, professors, and educators, with the goal of making complex subjects like chemistry accessible and understandable for all. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/
