The question is –
Is C2H2Br2 polar or nonpolar?
Answer:
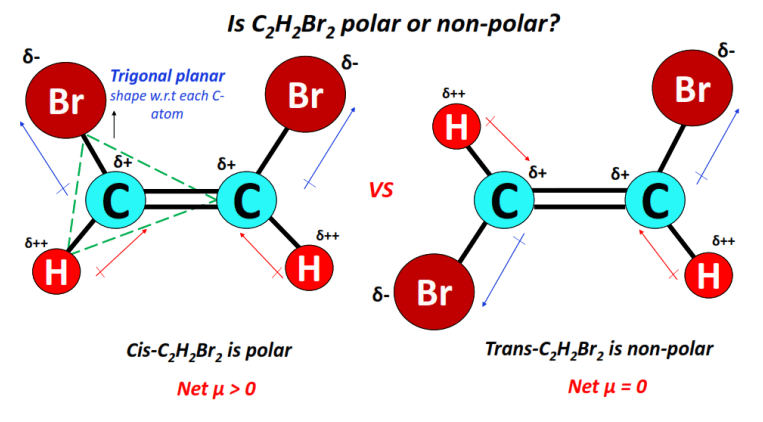
⇒ Cis-C2H2Br2 is polar while trans-C2H2Br2 is non-polar.
Explanation:
The polarity of C2H2Br2 depends on the arrangement of hydrogen and bromine atoms around the C=C double covalent bond.
1,2 dibromoethene (C2H2Br2) consists of a C=C double bond, two C-H single bonds, and two C-Br bonds.
The C=C bond is non-polar as it comprises of two identical carbon atoms having zero electronegativity differences.
A C-H bond is very weakly polar (almost non-polar as per Pauling’s electronegativity scale) as it consists of an electronegativity difference of only 0.35 units between the bonded carbon (E.N = 2.55) and hydrogen (E.N = 2.20) atoms.
Contrarily a C-Br bond is relatively more polar owing to an electronegativity difference of 0.41 units between the carbon and bromine (E.N = 2.96) atoms, respectively.
Each C-H bond thus possesses a small dipole moment value, while each C-Br bond has a relatively higher dipole moment value.
The shape of the molecule w.r.t each C-atom at the center is trigonal planar.
In cis-1,2-dibromoethene, the unequal dipole moments of C-H and C-Br bonds do not get canceled equally.
Thus it is overall polar (net µ > 0).
Contrarily, in trans-1,2-dibromoethene, the more electronegative Br-atoms are located directly opposite to each other.
Therefore, the C-H and C-Br dipole moments get canceled equally with the corresponding C-H and C-Br dipole moments, respectively, resulting in a non-polar molecule overall (net µ = 0).
Also, check –
⇒ How to identify polar or nonpolar compounds?

