The question is –
Is HCO3– polar or nonpolar?
Answer:
⇒ HCO3– is polar.
Explanation:
The bicarbonate (HCO3–) ion is polar as the unequal dipole moments of C-O (or C=O) and that of the O-H bond does not get canceled equally in the asymmetrical bent molecular shape and geometry.
HCO3– consists of two C-O single covalent bonds, one C=O double bond, and an O-H bond.
The C-O single or C=O double bond is polar as it consists of an electronegativity difference of 0.89 units between the covalently bonded carbon (E.N = 2.55) and oxygen (E.N = 3.44) atoms.
In contrast, the O-H bond is strongly polar as an even higher electronegativity difference of 1.24 units exists between an oxygen and a hydrogen (E.N = 2.20) atom.
Oppositely charged poles develop in HCO3– as the central C-atom and the terminal H-atom gains partial positive (δ+) charges while the three O-atoms gain partial negative (δ–) charges, respectively.
The shape of the molecular ion w.r.t central C-atom is trigonal planar while that w.r.t the O-H bonded O-atom is bent, angular, or V-shaped.
Two lone pairs of electrons present on the O-H bonded oxygen lead to strong lone pair-lone pair and lone pair-bond pair electronic repulsions, thus distorting the overall molecular shape and geometry.
The net dipole moment of two C-O bonds gets canceled to some extent with the dipole moment of a downwards-pointing C=O bond.
However, the strong dipole moment of an O-H bond stays uncancelled.
The charged electron cloud stays non-uniformly distributed, which leads to an overall polar HCO3– ion (net µ > 0).
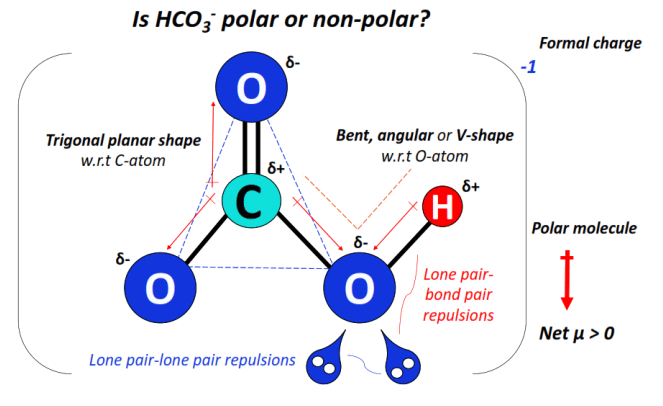
The C-O single-bonded oxygen atom (at the left) carries a -1 formal charge which is also the charge present on the bicarbonate ion overall.
Also, check –
⇒ How to identify polar or nonpolar compounds?
⇒ Is HCO3– an acid or base?

