Bohr model for all elements of the periodic table |Diagram + Chart|
Bohr model describes the visual representation of orbiting electrons around the small nucleus. It used different electron shells such as K, L, M, N…so on.
These shells hold a specific number of electrons, the electron shell which is closest to the nucleus has less energy and the electron shell which is farthest from the nucleus has more energy.
In this article, we will discuss the Bohr model for all elements of the periodic table.
Although the “Bohr model of the first 18 elements of the periodic table” or “Bohr model for the first 20 elements” is most important from the exam point of view, anyway, we will provide you the Bohr model for every element present in the periodic table.
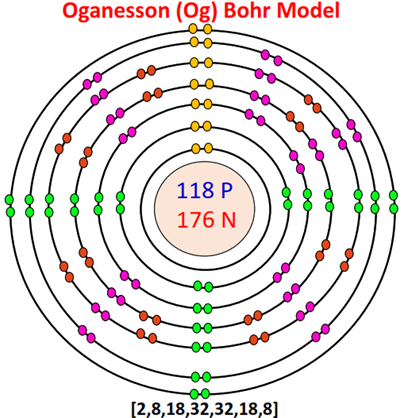
Bohr model for all elements [Diagrams]
That’s all, this is the list of Bohr diagrams for each element of the Periodic table. Also, if you find difficulty in drawing the Bohr model for any elements, then go through the links given below to understand the process of drawing the Bohr diagram in detail.
Go through the links to understand the process of drawing the Bohr model:-
- Scandium Bohr model
- Titanium Bohr model
- Vanadium Bohr model
- Oxygen Bohr model
- Boron Bohr model
- Beryllium Bohr model
- Lithium Bohr model
- Helium Bohr model
- Nitrogen Bohr model
- Fluorine Bohr model
- Neon Bohr model
- Carbon Bohr model
- Sodium Bohr model
- Silicon Bohr model
- Magnesium Bohr model
- Sulfur Bohr model
- Chlorine Bohr model
- Phosphorus Bohr model
- Aluminum Bohr model
- Argon Bohr model
- Potassium Bohr model
- Bromine Bohr model
- Calcium Bohr model
- Silver Bohr model
- Arsenic Bohr model
- Gold Bohr model
- Krypton Bohr model
- Iodine Bohr model
- Copper Bohr model
- Iron Bohr model
- Uranium Bohr model
- Nickel Bohr model
Also, check:
FAQ
What is the Bohr model of an element? |
| The Bohr model formally called the Bohr-Rutherford model is a visual representation of orbiting electrons around the small nucleus of an atom. |
How can you draw the Bohr model for each element of the Periodic table? |
To draw the Bohr model for an element, follow these basic steps.
|
Reference
LibreTexts. (2021). Electronic Structure of Atoms and Molecules: Bohr Diagrams of Atoms and Ions. In Physical and Theoretical Chemistry Textbook Maps: Supplemental Modules (Physical and Theoretical Chemistry). Retrieved from https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Electronic_Structure_of_Atoms_and_Molecules/Bohr_Diagrams_of_Atoms_and_Ions
About the author
Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. The team at Topblogtenz includes experts like experienced researchers, professors, and educators, with the goal of making complex subjects like chemistry accessible and understandable for all. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/





















































































































v