Sulfur (S) Orbital diagram, Electron configuration, and Valence electrons
Sulfur has an atomic number of 16 belongs to Group 16 also known as the Chalcogens family. It is situated in the P-block of the periodic table. Sulfur has the symbol S and “It is the tenth most abundant element by mass in the universe and the fifth most on Earth”.
In this article, we will discuss – Sulfur Orbital diagram, Electron configuration, and Valence electrons in detail.
Orbital diagram:- A orbital diagram is simply a pictorial representation of the arrangement of electrons in the orbital of an atom, it shows the electrons in the form of arrows, also, indicates the spin of electrons.
Electron configuration:- Electron configuration is the arrangement of electrons in atomic orbitals. It shows the electrons in numbers, It doesn’t show the details on the spin of electrons like the orbital diagram.
Valence electrons:- Valence electrons are the simply outermost electron of an atom situated in an outermost shell surrounding an atomic nucleus. They can participate in the formation of chemical bonds.
How to find Electron configuration of Sulfur (S)?
The electron configuration of Sulfur can be found using the Aufbau principle.
Aufbau Principle:
- The word ‘Aufbau’ in German means ‘building up’.
- The Aufbau rule simply gives the order of electrons filling in the orbital of an atom in its ground state.
- It states that the orbital with the lowest energy level will be filled first before those with high energy levels. In short, the electrons will be filled in the orbital in order of their increasing energies.
- For example, the 1s orbital will be filled first with electrons before the 2s orbital.
Simply understand that there are commonly four different types of subshells – s, p, d, and, f.
These subshells can hold a maximum number of electrons on the basis of a formula, 2(2l + 1) where ‘l’ is the azimuthal quantum number.
Value of ‘l’ for different subshells.
| Subshells | Value of ‘l’ | Maximum number of electrons, 2(2l + 1) | Number of orbitals in the subshell |
| s | 0 | 2 | 1 |
| p | 1 | 6 | 3 |
| d | 2 | 10 | 5 |
| f | 3 | 14 | 7 |
So, in short, the s subshell can hold a maximum of 2 electrons(1 orbital), the p subshell can hold 6 electrons(3 orbitals), the d subshell can hold 10 electrons(5 orbitals), and the f subshell can hold at most 14 electrons(7 orbitals).
Now, the electron configuration of an atom can be built by filling the electrons in a lower energy subshell first then higher, higher, and higher.
Generally, (n + l) rule is used to predict the energy level of subshells.
n = principle quantum number
l = Azimuthal quantum number
⇒ Lower the value of (n + l) for an subshell, the lower its energy, hence, it will be filled first with electrons.
⇒ For two different subshells having same (n + l) value, then the subshell with lower value of n has lower energy.
So, all these are basics of How filling of electrons will be done in different subshells, obviously, you don’t have so much time for writing electron configuration by using so many rules.
Therefore, we have a diagonal rule for electron filling order in the different subshells using the Aufbau principle.
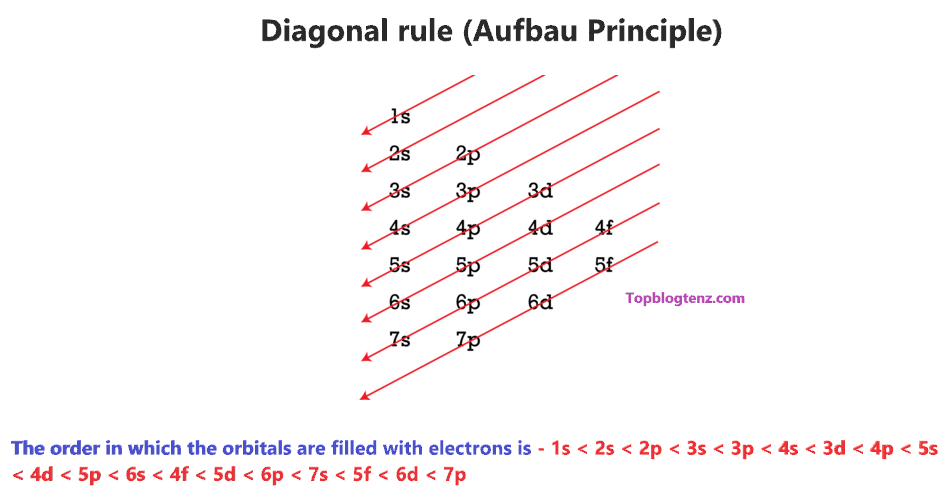
So, the order in which the orbitals are filled with electrons from lower energy to higher energy is – 1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s < 4f < 5d < 6p < 7s < 5f < 6d < 7p and so on.
Sulfur Electron configuration using the Aufbau Principle
- A Sulfur atom is a neutral atom that has an atomic number of 16 which implies it has a total of 16 electrons.
- As per the Aufbau rule, the electrons will be filled into 1s orbital first then 2s, then 2p…so on.
- Now, for the electron configuration of Sulfur, the first 2 electrons will go in 1s orbital since s subshell can hold a maximum of 2 electrons.
- The next two electrons will go into the 2s orbital, after that, the next 6 electrons will go into the 2p orbital since the p subshell can hold up to 6 electrons.
- The next two electrons will go into the 3s orbital, and after that, the remaining four electrons will go into the 3p orbital.
- Therefore, the electron configuration of Sulfur will be 1s22s22p63s23p4.

Sulfur (S) Electron Configuration
Check – Electron configuration calculator to count the electron configuration for any atom
Orbital diagram for Sulfur
The orbital diagram simply represents the arrangement of electrons in the different orbitals of an atom, it uses an arrow to represent the electrons, every orbital(one box) contains a maximum of 2 electrons.
There are three rules followed for constructing the orbital diagram for an atom.
(1). Aufbau’s principle:- This rule state that the lower energy orbital will be filled before the higher energy orbital, for example – the 1s orbital will fill before the 2s orbital.
(2). Hund’s rule:- This rule state that each orbital of a given subshell should be filled with one electron each before pairing them. That means “Each orbital gets one electron first, before adding the second electron to the orbital”.
(3). Pauli Exclusion Principle:- This rule state that, no two electrons can occupy the same orbital with the same spin. That means “One must be spin up (↑) and one must be spin down (↓)”.
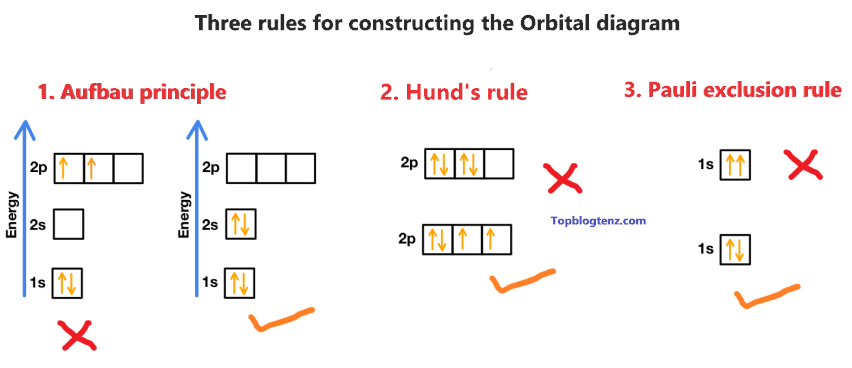
If you understand the above rules then constructing the orbital diagram or orbital notation for Sulfur is super easy.
Basics of Orbital diagram:-
There are different types of orbitals – s, p, d, and, f. These orbitals contain a number of boxes that can hold a number of electrons. Let’s see.
Each box will hold a maximum of 2 electrons with opposite spin.
- S orbital contains 1 box that can hold a maximum of 2 electrons.
- P orbital contains 3 boxes that can hold a maximum of 6 electrons.
- D orbital contains 5 boxes that can hold a maximum of 10 electrons.
- F orbital contains 7 boxes that can hold a maximum of 14 electrons.
The orbital diagram will also be filled with the same order as described by the Aufbau principle. (1s < 2s < 2p < 3s……and so on.)
Also check –
What is the Orbital diagram for Sulfur?
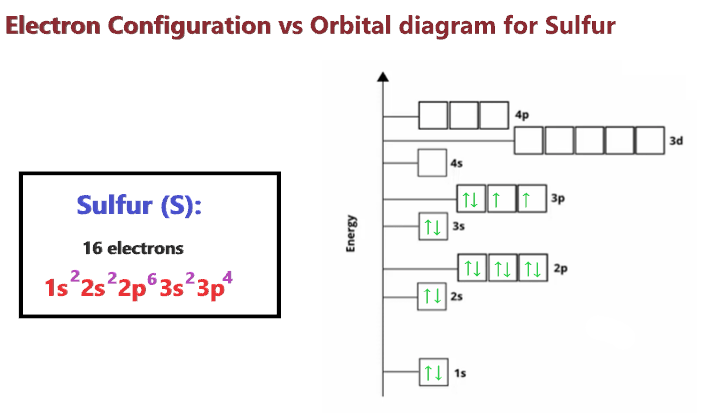
We know the electron configuration of Sulfur is 1s22s22p63s23p4, now for drawing its orbital diagram, we need to show its electrons in form of an arrow in different boxes using Hund’s and Pauli’s exclusion rule.
- The orbital diagram of Sulfur contains 1s orbital, 2s orbital, 2p orbital, 3s orbital, and 3p orbital. 1s orbital contains 1 box, 2s orbital also contains 1 box, 2p orbital contains 3 boxes, 3s orbital contains 1 box and 3p orbital contains 3 boxes.
- Sulfur has a total of 16 electrons and one box can hold up to two electrons.
- Therefore, the first two electrons will go into the 1s orbital, the next two will go into the 2s orbital, and after that, the next six electrons will go into the 2p orbital, since, the 2p orbital has 3 boxes.
- After that, the next two electrons will go into the 3s orbital, and the remaining four electrons will enter the 3p orbital, since, the 3p orbital has 3 boxes, so, these electrons will be filled using Hund’s rule. (Each box gets one electron first, then start pairing).

Sulfur Orbital diagram
Electron configuration Vs Orbital diagram for Sulfur
The main difference between the orbital diagram and electron configuration is an orbital diagram shows electrons in form of arrows whereas an electron configuration shows electrons in form of numbers. Also, the orbital diagram shows details on the spin of electrons whereas the electron configuration doesn’t show it.
Both these follow the Aufbau principle (Diagonal rule).
Also Read:–
Electron configuration for Sulfur via Bohr model (Orbit)
Bohr model describes the visual representation of orbiting electrons around the small nucleus. It used different electron shells such as K, L, M, N…so on.
These electron shells hold a specific number of electrons that can be calculated via the 2n2 formula where n represents the shell number.
| Electron shells | Shell number (n) | Max. number of electrons (2n2) |
| K | 1 | 2 |
| L | 2 | 8 |
| M | 3 | 18 |
| N | 4 | 32 |
So, K is the first shell or orbit that can hold up to 2 electrons, L is the 2nd shell which can hold up to 8 electrons, M is the third shell that can hold up to 18 electrons, and N is the fourth shell that can hold up to 32 electrons.
Now, Sulfur has an atomic number of 16 and it contains a total number of 16 electrons. Hence, 2 electrons will go in the first shell(K), 8 electrons will go in the second shell(L), and the remaining six electrons will go in the third shell(M).
Therefore, the electrons per shell for Sulfur are 2, 8, 6, hence, we can say, based on the shell, the electronic configuration of the Sulfur atom is [2, 8, 6].
Also check – How to draw Bohr model of Sulfur atom
Sulfur Valence electrons
Valence electrons are the outermost electrons present in the outermost shell of an atom. They have more energy, hence, they are part of most chemical reactions.
We can find valence electrons of an atom either by knowing its periodic group number or its electron configuration. Both these ways are super easy.
Finding Sulfur Valence electrons through the Group number
For neutral atoms, the valence electrons of an atom will be equal to its main periodic group number. However, for transition metals, the process of finding valence electrons is complicated.
Now, for determining the valence electron for the Sulfur atom, look at the periodic table and find its Group number. The group number can be found from its column on the periodic table.

So, the number of valence electrons in Sulfur is 6. Since it belongs to Group 16th or 6A in the Periodic table.
Finding Sulfur Valence electrons through the Electron configuration or Bohr model
We know, the electron configuration of the Sulfur atom is 1s22s22p63s23p4, and valence electrons are those electrons found in the outer shell of an atom.
This electron configuration of Sulfur shows that the outer shell of Sulfur has 6 electrons(3s23p4), hence, the number of valence electrons in the Sulfur atom is 6.
Also, we know, the electron configuration of Sulfur, based on the shells is [2, 8, 6], which means, that two electrons are present in the first shell, eight electrons are present in the 2nd shell, and six electrons are present in the third shell or outer shell.
Hence, the electrons found in the 3rd shell of the Sulfur atom are its valence electrons because it is the outermost shell also called the valence shell.
The 3rd shell or outer shell of the Sulfur atom contains 6 electrons, therefore, the number of valence electrons in the Sulfur atom is 6.

Check – Valence electron calculator to calculate the number of valence electrons for any atom
Electron configuration, Valence electrons, and Orbital diagram of Sulfur in tabular form
| Name of atom | Sulfur (S) |
| Number of electrons | 16 |
| Number of electrons per shell | [2, 8, 6] |
| Number of valence electrons | 6 |
| Electron configuration | 1s22s22p63s23p4 or [Ne] 3s23p4 |
| Orbital diagram | Consists of five orbitals – 1s, 2s, 2p, 3s, and 3p. |
Also read:
- Nitrogen orbital diagram and electron configuration
- Oxygen orbital diagram and electron configuration
- Carbon orbital diagram and electron configuration
- Fluorine orbital diagram and electron configuration
- Neon orbital diagram and electron configuration
- Boron orbital diagram and electron configuration
- Sodium orbital diagram and electron configuration
- Magnesium orbital diagram and electron configuration
- Aluminum orbital diagram and electron configuration
- Silicon orbital diagram and electron configuration
- Phosphorous orbital diagram and electron configuration
- Chlorine orbital diagram and electron configuration
- Argon orbital diagram and electron configuration
- Potassium orbital diagram and electron configuration
- Calcium orbital diagram and electron configuration
- Beryllium orbital diagram and electron configuration
- Lithium orbital diagram and electron configuration
FAQ
What are the Ground state and Excited-state Electron configurations of Sulfur?There is a simple difference between Ground state and Excited-state configuration. The ground state configuration of an atom is the same as its regular electron configuration in which electrons remain in the lowest possible energy. So, the ground-state electron configuration for the Sulfur atom is 1s22s22p63s23p4. The excited-state configuration of an atom is different from the regular configuration of an atom, this occurs, when an electron is excited and jumps into a higher orbital. The excited-state electron configuration for Sulfur is 1s22s22p63s23p33d1. |
What is the shorthand electron configuration of Sulfur?The shorthand electron configuration for the Sulfur atom is [Ne] 3s23p4. ∴ [Ne] electron configuration is 1s22s22p6. |
Which element has the 1s22s22p63s23p4 Electron configuration?Element with electron configuration 1s22s22p63s23p4 is Sulfur (s) that has the atomic number of 16. |
How many valence electrons does Sulfur have?The Sulfur atom has 6 valence electrons in its outermost or valence shell. Sulfur is belonged to group 16th or 6A and has the atomic number of 16. |
What is the orbital diagram for Sulfur (S)?The orbital diagram for Sulfur is drawn with 5 orbitals. The orbitals are 1s, 2s, 2p, 3s, and 3p. The Sulfur orbital diagram contains 2 electrons in the 1s orbital, 2 electrons in the 2s orbital, the six electrons in the 2p orbital, the two electrons in the 3s orbital, and the remaining four electrons in the 3p orbital. An orbital diagram for a ground-state electron configuration of a Sulfur atom is shown below-
|
What is the electron configuration of the S2- ion?We know, in general, that the electron configuration of Sulfur (S) is 1s22s22p63s23p4. Now, in the S2- ion, the negative charge means, Sulfur gains two electrons. Therefore, to write the electron configuration of the S2- ion, we have to add two electrons to the configuration of Sulfur (S). ∴ The resulting electron configuration for the Sulfide ion (S2-) will be 1s22s22p63s23p6. It resembles the configuration of the nearest inert gas i.e Argon. |
Properties of Sulfur
- It is multivalent and nonmetallic in nature.
- It appears as bright yellow and crystalline solid at room temperature.
- Its oxidation state varies from -2 to +6.
- It has a boiling point of 444.6 °C and a melting point of 115.21 °C.
- Its electronegativity is 2.58.
- It can form several polyatomic molecules.
- It has an orthorhombic crystal structure.
- Sulfur is insoluble in water.
Summary
- The electron configuration of Sulfur in terms of the shell or orbit is [2, 8, 6].
- The ground-state electron configuration of the Sulfur (S) atom is 1s22s22p63s23p4. And for the excited state, it is 1s22s22p63s23p33d1.
- The shorthand electron configuration for Sulfur is [Ne] 3s23p4.
- The electron configuration for the Sulfide ion (S2-) is 1s22s22p63s23p6.
- The number of valence electrons available for the Sulfur atom is 6. Sulfur is situated in Group 16th or 6A and has an atomic number of 16.
- The first shell of Sulfur has 2 electrons and the outer shell or valence shell of Sulfur has 6 electrons, hence, the number of valence electrons in the Sulfur atom is 6.
- The orbital diagram for Sulfur is drawn by following three principles – the Aufbau principle, Hund’s principle, and Pauli’s exclusion principle.
- The Sulfur orbital diagram comprises five orbitals. The five orbitals are 1s, 2s, 2p, 3s, and 3p.
- The first two electrons will go in the 1s orbital, the next two in the 2s orbital, the next six in the 2p orbital, the next two electrons in the 3s orbital, and the remaining four electrons in the 3p orbital.
About the author
Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. The team at Topblogtenz includes experts like experienced researchers, professors, and educators, with the goal of making complex subjects like chemistry accessible and understandable for all. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/




Interesting post! I’m curious about the electron configuration and valence electrons.