Iodine (I) Bohr Model
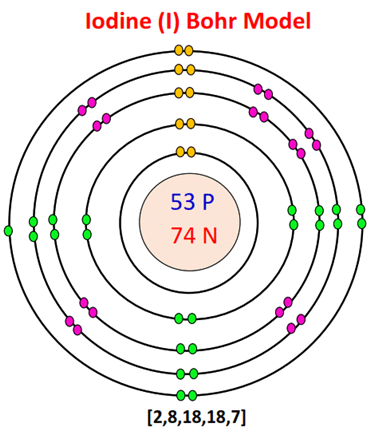
- The Bohr Model of Iodine (I) has a nucleus that contains 74 neutrons and 53 protons.
- This nucleus is surrounded by five electron shells.
- The first shell of the Bohr diagram of Iodine has 2 electrons, the 2nd shell has 8, the 3rd shell has 18, the 4th has 18, and the 5th shell has 7 electrons.
| Name | Iodine Bohr Model |
| Number of neutrons | 74 |
| Number of protons | 53 |
| Number of electrons | 53 |
| Total electron shells | 5 |
| Electron in the First shell | 2 |
| Electrons in the Second shell | 8 |
| Electrons in the Third shell | 18 |
| Electrons in the Fourth shell | 18 |
| Electrons in the Fifth shell | 7 |
Also check –
Did you like it?
Related Questions
- What is the Bohr model for Hydrogen? – Chemistry QnA
- What is the Bohr model for Helium? – Chemistry QnA
- What is the Bohr model for Lithium? – Chemistry QnA
- What is the Bohr model for Beryllium? – Chemistry QnA
- What is the Bohr model for Boron? – Chemistry QnA
- What is the Bohr model for Carbon? – Chemistry QnA
- What is the Bohr model for Nitrogen? – Chemistry QnA
- What is the Bohr model for Oxygen? – Chemistry QnA
- What is the Bohr model for Fluorine? – Chemistry QnA
- What is the Bohr model for Neon? – Chemistry QnA
- What is the Bohr model for Sodium? – Chemistry QnA
- What is the Bohr model for Magnesium? – Chemistry QnA
- What is the Bohr model for Aluminum? – Chemistry QnA
- What is the Bohr model for Silicon? – Chemistry QnA
- What is the Bohr model for Phosphorus? – Chemistry QnA
- What is the Bohr model for Sulfur? – Chemistry QnA
- What is the Bohr model for Chlorine? – Chemistry QnA
- What is the Bohr model for Argon? – Chemistry QnA
- What is the Bohr model for Potassium? – Chemistry QnA
- What is the Bohr model for Calcium? – Chemistry QnA
- What is the Bohr model for Scandium? – Chemistry QnA
- What is the Bohr model for Titanium? – Chemistry QnA
- What is the Bohr model for Vanadium? – Chemistry QnA
- What is the Bohr model for Chromium? – Chemistry QnA
- What is the Bohr model for Manganese? – Chemistry QnA
- What is the Bohr model for Iron? – Chemistry QnA
- What is the Bohr model for Cobalt? – Chemistry QnA
- What is the Bohr model for Nickel? – Chemistry QnA
- What is the Bohr model for Copper? – Chemistry QnA
- What is the Bohr model for Zinc? – Chemistry QnA
- What is the Bohr model for Gallium? – Chemistry QnA
- What is the Bohr model for Germanium? – Chemistry QnA
- What is the Bohr model for Arsenic? – Chemistry QnA
- What is the Bohr model for Selenium? – Chemistry QnA
- What is the Bohr model for Palladium? – Chemistry QnA
- What is the Bohr model for Bromine? – Chemistry QnA
- What is the Bohr model for Krypton? – Chemistry QnA
- What is the Bohr model for Rubidium? – Chemistry QnA
- What is the Bohr model for Strontium? – Chemistry QnA
- What is the Bohr model for Yttrium? – Chemistry QnA
- What is the Bohr model for Zirconium? – Chemistry QnA
- What is the Bohr model for Niobium? – Chemistry QnA
- What is the Bohr model for Molybdenum? – Chemistry QnA
- What is the Bohr model for Technetium? – Chemistry QnA
- What is the Bohr model for Ruthenium? – Chemistry QnA
- What is the Bohr model for Rhodium? – Chemistry QnA
- What is the Bohr model for Silver? – Chemistry QnA
- What is the Bohr model for Cadmium? – Chemistry QnA
- What is the Bohr model for Indium? – Chemistry QnA
- What is the Bohr model for Tin? – Chemistry QnA
- What is the Bohr model for Antimony? – Chemistry QnA
- What is the Bohr model for Tellurium? – Chemistry QnA
- What is the Bohr model for Xenon? – Chemistry QnA
- What is the Bohr model for Caesium? – Chemistry QnA
- What is the Bohr model for Barium? – Chemistry QnA
- What is the Bohr model for Lanthanum? – Chemistry QnA
- What is the Bohr model for Cerium? – Chemistry QnA
- What is the Bohr model for Praseodymium? – Chemistry QnA
- What is the Bohr model for Neodymium? – Chemistry QnA
- What is the Bohr model for Promethium? – Chemistry QnA
- What is the Bohr model for Samarium? – Chemistry QnA
- What is the Bohr model for Europium? – Chemistry QnA
- What is the Bohr model for Gadolinium? – Chemistry QnA
- What is the Bohr model for Terbium? – Chemistry QnA
- What is the Bohr model for Dysprosium? – Chemistry QnA
- What is the Bohr model for Holmium? – Chemistry QnA
- What is the Bohr model for Erbium? – Chemistry QnA
- What is the Bohr model for Thulium? – Chemistry QnA
- What is the Bohr model for Ytterbium? – Chemistry QnA
- What is the Bohr model for Lutetium? – Chemistry QnA
- What is the Bohr model for Hafnium? – Chemistry QnA
- What is the Bohr model for Tantalum? – Chemistry QnA
- What is the Bohr model for Tungsten? – Chemistry QnA
- What is the Bohr model for Rhenium? – Chemistry QnA
- What is the Bohr model for Osmium? – Chemistry QnA
- What is the Bohr model for Iridium? – Chemistry QnA
- What is the Bohr model for Platinum? – Chemistry QnA
- What is the Bohr model for Gold? – Chemistry QnA
- What is the Bohr model for Mercury? – Chemistry QnA
- What is the Bohr model for Thallium? – Chemistry QnA
- What is the Bohr model for Lead? – Chemistry QnA
- What is the Bohr model for Bismuth? – Chemistry QnA
- What is the Bohr model for Polonium? – Chemistry QnA
- What is the Bohr model for Astatine? – Chemistry QnA
- What is the Bohr model for Radon? – Chemistry QnA
- What is the Bohr model for Francium? – Chemistry QnA
- What is the Bohr model for Radium? – Chemistry QnA
- What is the Bohr model for Actinium? – Chemistry QnA
- What is the Bohr model for Thorium? – Chemistry QnA
- What is the Bohr model for Protactinium? – Chemistry QnA
- What is the Bohr model for Uranium? – Chemistry QnA
- What is the Bohr model for Neptunium? – Chemistry QnA
- What is the Bohr model for Plutonium? – Chemistry QnA
- What is the Bohr model for Americium? – Chemistry QnA
- What is the Bohr model for Curium? – Chemistry QnA
- What is the Bohr model for Berkelium? – Chemistry QnA
- What is the Bohr model for Californium? – Chemistry QnA
- What is the Bohr model for Einsteinium? – Chemistry QnA
- What is the Bohr model for Fermium? – Chemistry QnA
- What is the Bohr model for Mendelevium? – Chemistry QnA
- What is the Bohr model for Nobelium? – Chemistry QnA
- What is the Bohr model for Lawrencium? – Chemistry QnA
- What is the Bohr model for Rutherfordium? – Chemistry QnA
- What is the Bohr model for Dubnium? – Chemistry QnA
- What is the Bohr model for Seaborgium? – Chemistry QnA
- What is the Bohr model for Bohrium? – Chemistry QnA
- What is the Bohr model for Hassium? – Chemistry QnA
- What is the Bohr model for Meitnerium? – Chemistry QnA
- What is the Bohr model for Darmstadtium? – Chemistry QnA
- How to draw Roentgenium bohr model? – Chemistry QnA
- How to draw Copernicium bohr model? – Chemistry QnA
- How to draw Nihonium bohr model? – Chemistry QnA
- How to draw Flerovium bohr model? – Chemistry QnA
- How to draw Moscovium bohr model? – Chemistry QnA
- How to draw Livermorium bohr model? – Chemistry QnA
- How to draw Tennessine bohr model? – Chemistry QnA
- How to draw Oganesson bohr model? – Chemistry QnA
About the author
Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. The team at Topblogtenz includes experts like experienced researchers, professors, and educators, with the goal of making complex subjects like chemistry accessible and understandable for all. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/
