How to know if compound is acid or base or neutral or salt? - Nature of solution

In chemistry, we get these terms frequently such as “Acid”, “Base”, “Salt”. “Amphoteric”. And somewhere you really think that whether the given compound is acid or base or salt or neutral. So, in this article, I will try to clear your all doubt regarding the identification of acid and base.
First of all, Let’s try to learn the basic definition of acid, base, or salt, then, we will move to their concept.
Acid = Acid is something that has a pH value lower than 7 and has the ability “to donate the proton” or “to accept a pair of electrons”.
Base = It is something that has a pH value greater than 7 and has the ability of “accepting the proton” or “to donating the pair of electrons”.
Salt = It is an ionic compound that is made up of a cation and anion. Salt is obtained by the neutralization of an acid with a base.
Amphoteric = An amphoteric compound is one that can react with both acids and bases, depends on the reactive compound means, it acts as a base in presence of acid and act as the acid in presence of a base.
The best way to tell if something is an acid or base to look at following condition –
- If compound releases more number of H+ ions in aqueous solution, it is acidic.
- And if the compound releases more number of OH- ions in aqueous solution, it is basic.
Or the best way to know if the compound is an acid or base
|
How to tell if something is an acid or base?
The best way to tell whether the compound is acid or base is by applying the three different definitions of acid and base given by Arrhenius, Bronsted-Lowry, and Lewis’s theory.
Arrhenius theory: According to this, a compound is an acid when it furnishes an H+ ion on dissolving in an aqueous solution or increases the concentration of hydrogen ion in the solution.
And a compound is said to be base when it furnishes OH– ions on dissolving in an aqueous solution or increases the concentration of OH– ions in solution.
Example – HCl, HCN, CH3COOH, HNO3, HNO2, HClO4, HCOOH, H3PO4, HI, HBr, etc. All these compounds are Arrhenius acid in nature as they release H+ ions when dissolved in an aqueous solution, hence, increasing the concentration of H+ ions.
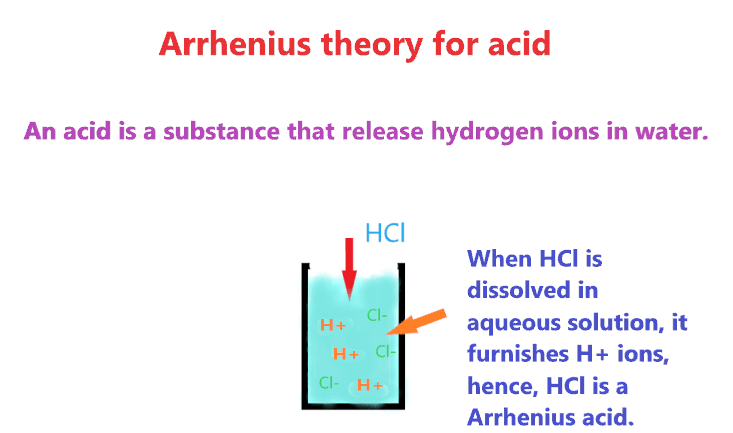
Also check-
And the compound such as NaOH, KOH, LiOH, Ba(OH)2, Ca(OH)2, etc. is the Arrhenius base as they release OH– ions when dissolved in an aqueous solution, hence, increasing the concentration of OH– ions.
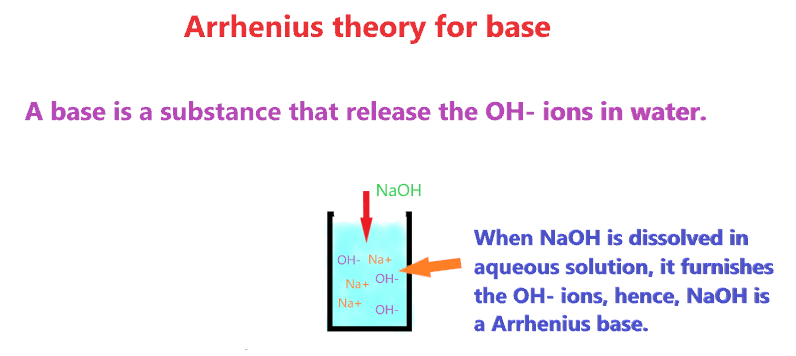
Also check:
Note: – Arrhenius’s theory is limited to the aqueous solution, which means, the presence of water solution is a must for knowing the acidic or basic nature of the substance.
So, for overcoming this problem, another theory is proposed named the Bronsted-Lowry theory which can explain the nature of the compound in both aqueous and non-aqueous solvents.
Bronsted-Lowry theory: This theory is a generalization of the Arrhenius theory. According to this, the substance which donates the proton to another species is classified as a Bronsted-Lowry acid whereas the substance which accepts the proton from another species is classified as a Bronsted-Lowry base.
- The Bronsted-Lowry theory is an acid-base reaction theory that means a substance can only act as an acid in presence of a base similarly a substance can only act as a base in presence of an acid.
- When acid and base react with each other, the acid compound donates its proton and forms a base, called the conjugate base of the acid, Similarly, the base compound accepts the proton and forms an acid, called the conjugate acid of the base.
The reaction defines the concept of the Bronsted-Lowry theory.
⇒ Acid + Base ⇌ Conjugate Base + Conjugate Acid
Let’s understand it with an example of HCl reacting with NH3.
So, HCl is acid and NH3 is a base, therefore, the Bronsted-Lowry theory is applicable here.
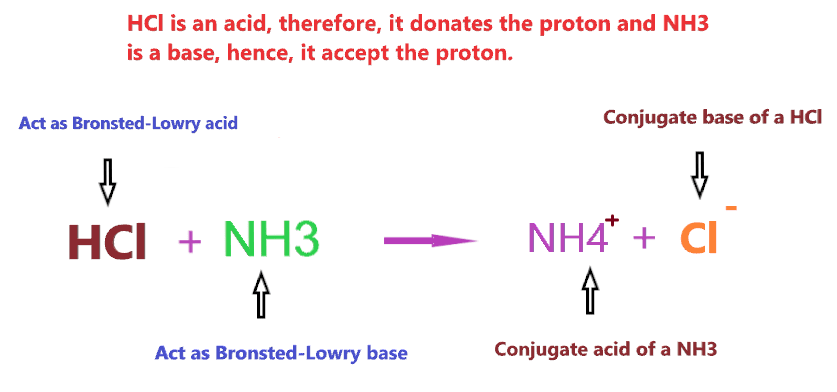
So, HCl as an acid donates its proton and makes a conjugate base(Cl–), and NH3 as a base accept the proton and makes a conjugate acid(NH4+).
Therefore, we can say, HCl acts as a Bronsted-Lowry acid, and NH3 acts as a Bronsted-Lowry base when reacts with each other.
Let’s take another example, CH3COOH reacting with the H2O.
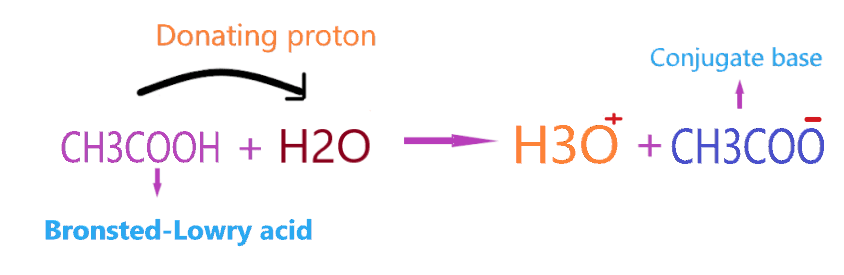
When CH3COOH reacts with water, it donates the proton to the water molecule and makes a conjugate base(CH3COO–) whereas H2O accepts the proton and makes the conjugate acid(H3O+).
Therefore, CH3COOH acts as a Bronsted-Lowry acid, and H2O acts as a Bronsted-Lowry base when they react with each other.
That’s it, you can apply this theory in a reaction that contains both acid and base and can easily predict their conjugate acid and base.
Limitation of Bronsted-Lowry theory: –
“Bronsted Lowry’s theory is limited to explaining the acidity and basicity of only protons containing compounds.”
There is no absolute acid and base defined by this theory, the substance can only act as the acid in presence of a base similarly the substance can only act as a base in presence of acid.
The same compound that acts as an acid in one reaction can act as a base in another reaction, it all depends on the strength of the reactive compound.
As we know, HCl is a strong acid and it can easily donate the proton when reacts with bases like NH3, NaOH, etc., or weak acidic like CH3COOH, HCO3–, etc. Hence, HCl act here as a Bronsted-Lowry acid.
But when HCl reacts with superacids like Fluoroantimonic acid, then it will act as a base, because superacids are able to donate the proton thousands of times better than HCl, H2SO4, etc.
So, Please consider the strength of the substance when applying the Bronsted-Lowry theory.
In short, you have to remember is that no compound is absolute acid or base, all these can act as an acid or base depending on the circumstances.
Lewis theory: This theory used electrons instead of proton transfer. According to the lewis theory, a compound is said to be acid when it accepts the electron pair and a compound is said to be base when it donates the electron pair.
Lewis acid → lone pair acceptor
Lewis base → lone pair donator
⇒ Lewis acid: They are electron-loving compounds and are also known as electrophilic compounds that accept the electron pair from Lewis bases.
Some examples of Lewis acids that can accept the pair of electrons from another species.
- The molecules or ions with an incomplete octet of electrons. Example – BF3, BCl3, AlF3, etc.
- The molecule that central atom has an empty d-orbital. Example- SiCl4 (1s22s22p63s23p2 3d0).
- The compounds that the central atom formed multiple bonds with the adjacent atoms. Example – SiO2 (O = Si = O), CO2, SO2, etc.
- Simple cations like H+, Na+, Mg+2, Al+3, etc. are lewis acids since they are able to accept the electrons. ( Exception NH4+, PH4+ are not a lewis acid)
⇒ Lewis base: They are electron-rich species and also known as nucleophile compounds that donate the electron pairs to the Lewis acids.
Some examples of Lewis bases that can donate the pair of electrons to other species.
- The negative ion such as H–, OH–, F–, etc.
- Species that have lone pair of electrons. Example – NH3, H2O, CH3–, etc.
Let’s understand it with the help of an example-

The electrons in the hydrogen-fluorine bonds attracted towards the fluoride ion because of it high electronegativity leaving hydrogen slightly positive and fluorine negative.
The lone pair on the nitrogen of NH3 molecule attracted towards hydrogen atom in HF molecule.
As it approaches it, the electrons in the hydrogen-fluorine bond are repelled still further towards the fluorine.
Eventually, a co-ordinate bond is formed between the nitrogen and the hydrogen, and the fluorine breaks away as a fluoride ion.
The whole HF molecule acts as Lewis acid as it accept the lone pair from nitrogen atom, and in this process it breaks up.
So, HF accepts the lone pair of the electron, therefore, it is Lewis acid and NH3 donates the lone pair of the electron, therefore, it is Lewis base.
Can you tell if the compound is acid or base by looking at their formula?
Some of you have misconceptions that H in the chemical formula indicates acid and OH indicates base but it is totally wrong.
An acid is a compound that furnishes some H+ ions and a base is a compound that furnishes some OH– ions, so, it’s not that a compound contains H and it must be acidic, similarly, a compound contains OH group and it must be basic, No that’s totally wrong.
The compound should release H+ and OH– ions, in order to be considered acidic or basic.
“It is not the OH hydroxy group that is basic but the OH- hydroxide ion. Only those compounds that dissociate into some cation and a hydroxide ion can be considered basic”.
Similarly, it’s not that H group that is acidic but the H+ ion. Only those compound that dissociate into some anion and H+ can be considered a acidic.
For example – HCl, HNO3, HClO4, HI, HF, etc. are acid in nature because they release H+ ions when dissolved in an aqueous solution, it’s not that they contain the H group and that’s why they are acidic.
Similarly, CH3COOH contains an OH group in its formula but it is not basic because it releases H+ ions on dissociation, hence, CH3COOH is an acid despite containing the OH group.
One more example is CH3OH, many of you already assume it as a base because it contains the OH group, but no, CH3OH is slightly acidic because it has one hydrogen atom attached to the oxygen atom which can be dissociated as H+ ions in the aqueous solution.
Also, the NH3 doesn’t have an OH group, still, it is a base because, in water solution, it has a small tendency to attract a proton, giving NH4+ and OH–.
So, there is no direct formula or you can’t just predict the acid or base by looking at their chemical formula.
Also read:-
How to tell if a salt is acidic or basic or neutral?
In chemistry, salt is a substance obtained by the neutralization of acid compounds with base compounds. Salt is made up of a cation(base) and anion(acid), the bond between cation and anion is ionic, hence it is also called an ionic compound.

There are three types of salts formed in the neutralization reaction “Acidic salt”, “Basic salt”, and “Neutral salt”.

Acidic salt: Acidic salt is formed when a neutralization reaction carries out between strong acid and weak base.
For example – NH4NO3 is an acidic salt because it is formed when a neutralization reaction carries out between the weak base(NH4OH) and strong acid(HNO3).

Also Read:-
Basic salt: A basic salt is formed when a neutralization reaction carries out between a strong base and weak acid.
For example – Na2CO3 is a basic salt as it is formed when a stronger base(NaOH) reacts with the weaker acid(H2CO3).

Also Read:-
Neutral salt: This salt has a pH value equal to 7 which means it neither has acidic properties nor basic properties. It is formed when a neutralization reaction carries out between a strong acid and strong base or weak acid and weak base.
For example – NaNO3 is a neutral salt that is made up by the neutralization of a strong base(NaOH) with a strong acid(HNO3).

Also Read:-
How to know if the solution is acidic or basic or neutral?
A solution nature depends on the H+ and OH– ions. When more OH– ions are present in a solution, then the solution becomes basic, and when more H+ ions are present in a solution, the solution becomes acidic.
And when the H+ and OH– are equal in number then the nature of the solution will be neutral.
The important concept of acid-base strength–
- A stronger base + weaker acid forms a basic aqueous solution due to the presence of more OH– ions. (OH– > H+)
- Stronger acid + weaker base forms an acidic aqueous solution due to the presence of more H+ ions. (H+ > OH–)
- Stronger/weaker acid + Stronger/weaker base forms a neutral aqueous solution due to the presence of the same number of OH– and H+ in the solution. (H+ = OH–)
Let’s take an example of a Na2CO3 aqueous solution to understand this concept-
When sodium carbonate is dissolved in water, it dissociates into two ions Na+ and CO32-.
⇒ Na2CO3 → 2Na+ + CO32-
The Na+ is a cation of strong base (NaOH), hence, it will not undergo hydrolysis in water and doesn’t affect the pH of the solution. But the CO32- is the anion of a weak acid (H2CO3) that gets hydrolysis in water to form bicarbonate ions(HCO3–), leaving OH– ions that make the solution basic.
⇒ CO32- + H2O → HCO3– + OH–
The bicarbonate ion(HCO3–) remains mostly unionized in the solution, hence, the final solution contains more OH– ion than H+ ion.

Therefore, with the presence of additional OH– ions in the aqueous solution, sodium carbonate (Na2CO3) solution becomes basic in nature.
How to know if compound is a Amphoteric?
“Amphoteric means the compound has the ability to act as an acid with a base and base with an acid”.
Examples of Amphoteric compounds – H2O, NaHCO3, HCO3–, CH3OH, etc.
Please go through these links to know-
Summary
- To determine whether a compound is an acid or base, you can apply the three important acid-base theories, Arrhenius, Bronsted-Lowry, and Lewis’s theory.
- To predict whether the solution is acidic or basic, you can check its pH level, when the concentration of H+ ions is more than OH– ions, then the solution will be acidic with a pH value less than 7, and when the concentration of OH– ions is more than the H+ ions in solution, then solution will be basic with pH value more than 7.
- A compound is said to be acid that “releases H+ ions in aqueous solution” or “donate the proton to other compounds” or “Accept the electron pairs”
- A compound is said to be a base that “release OH– ions in aqueous solution” or “accept the proton from another compound” or “Donate the electron pairs”.
- To know if salt is acidic or basic or neutral, consider the weightage of the strong compound, if the salt is prepared by strong acid and weak base, then the salt is acidic in nature, if the salt is prepared by the strong base and a weak acid, then the salt is basic in nature and when the salt is prepared by both strong acid and strong base, then the salt is neutral in nature.
About the author
Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. The team at Topblogtenz includes experts like experienced researchers, professors, and educators, with the goal of making complex subjects like chemistry accessible and understandable for all. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/
