Full electron configuration for every element in the periodic table |118 elements|
Welcome to our comprehensive guide on the electron configurations of all 118 elements in the periodic table.
In this article, we’ve organized all the elements of the periodic table by atomic number with their name and provided their full electron configurations in a clear and easy-to-read tabular format.
| Electron configuration for every element in the Periodic table (118 elements) | ||
| # | Name of Element | Electronic Configuration |
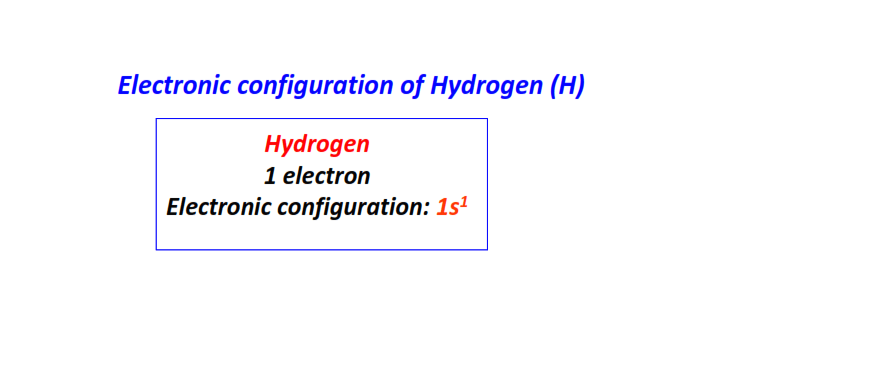
| 1 | Hydrogen (H) | 1s1
|
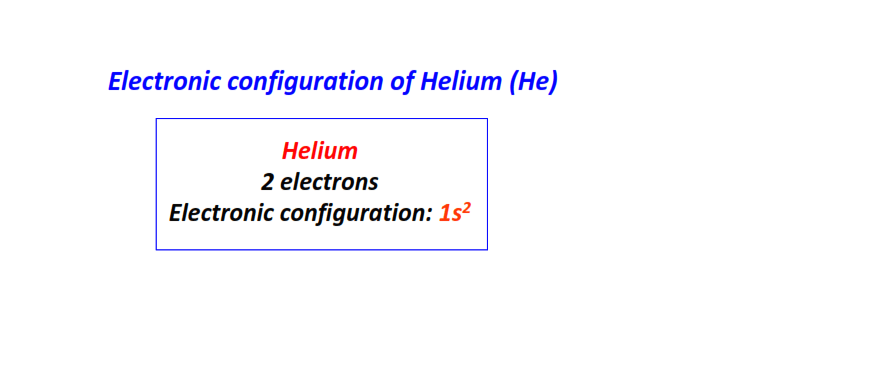
| 2 | Helium (He) | 1s2
|
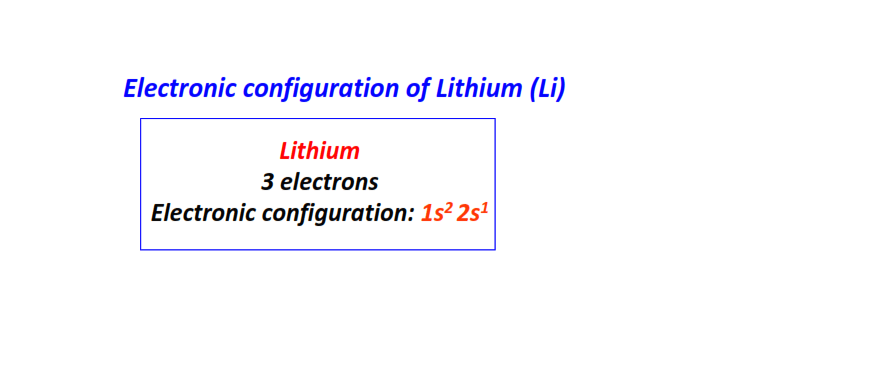
| 3 | Lithium (Li) | 1s22s1
|
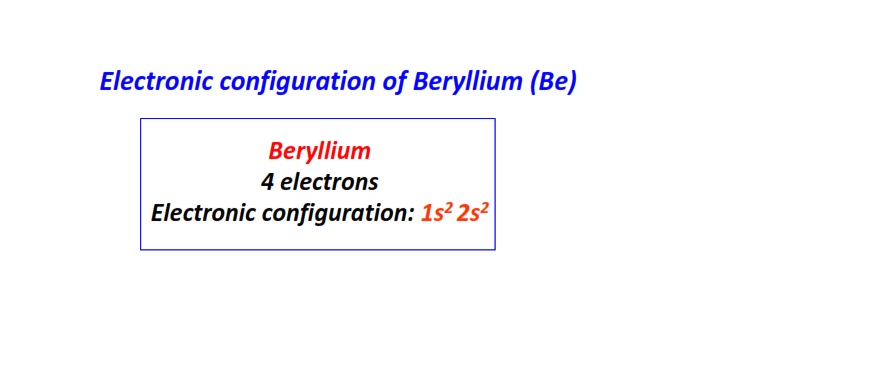
| 4 | Beryllium (Be) | 1s22s2
|
| 5 | Boron (B) | 1s22s22p1 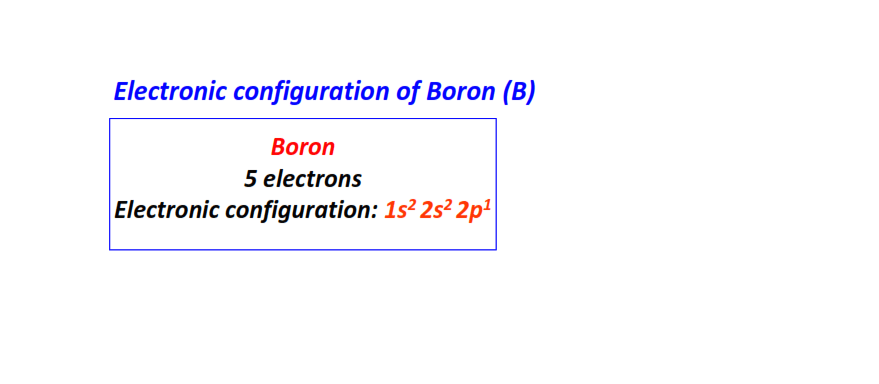 |
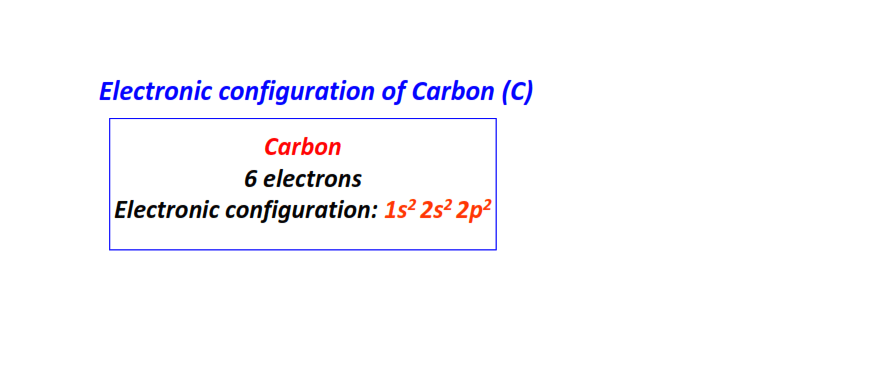
| 6 | Carbon (C) | 1s22s22p2
|
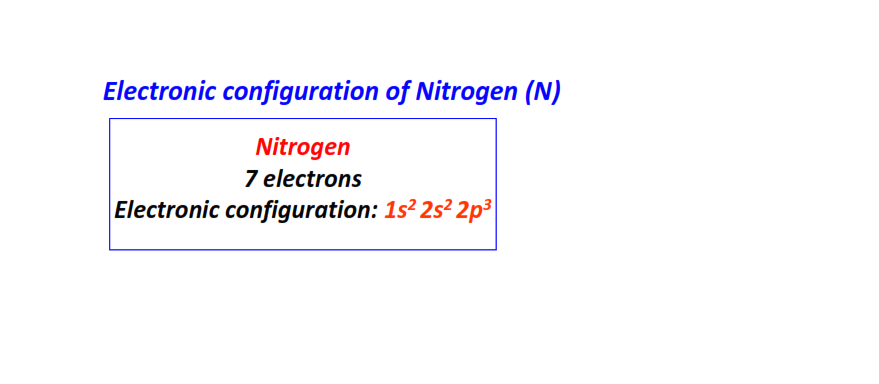
| 7 | Nitrogen (N) | 1s22s22p3
|
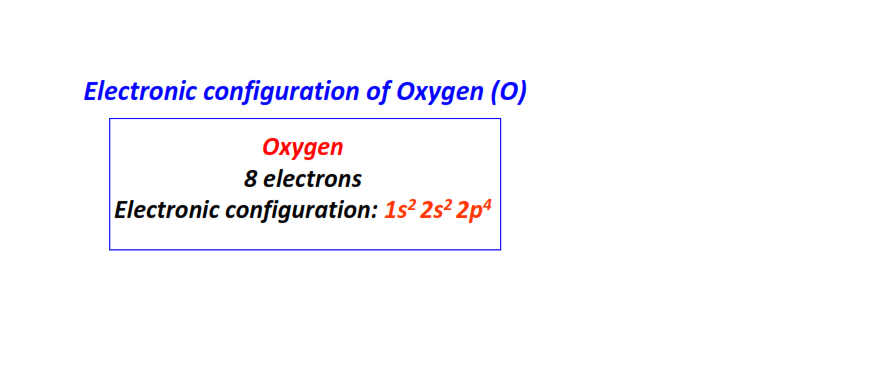
| 8 | Oxygen (O) | 1s22s22p4
|
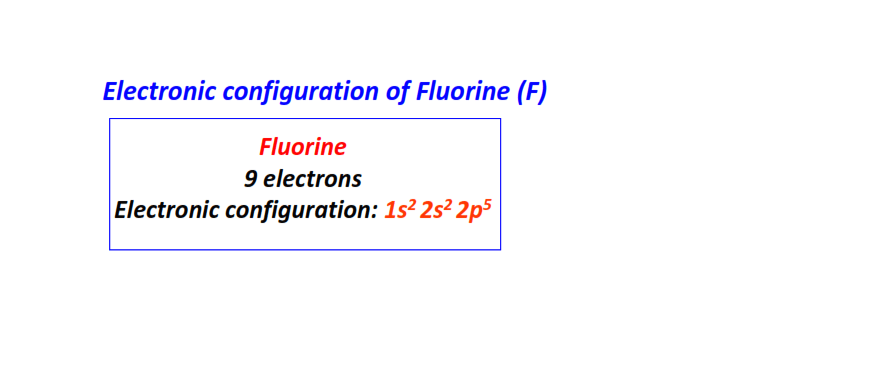
| 9 | Fluorine (F) | 1s22s22p5
|
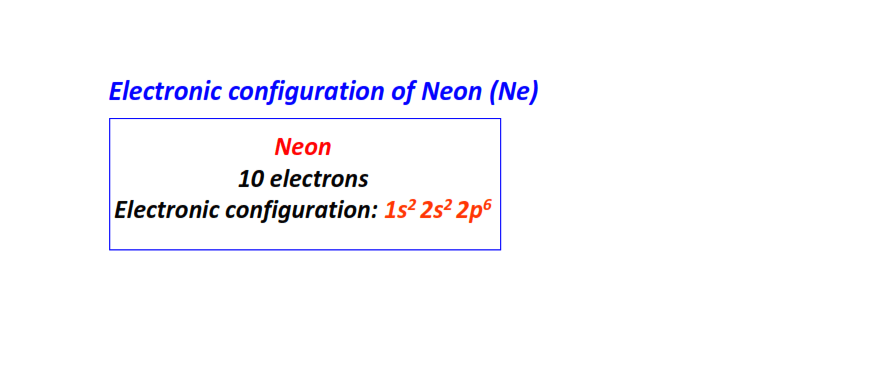
| 10 | Neon (Ne) | 1s22s22p6
|
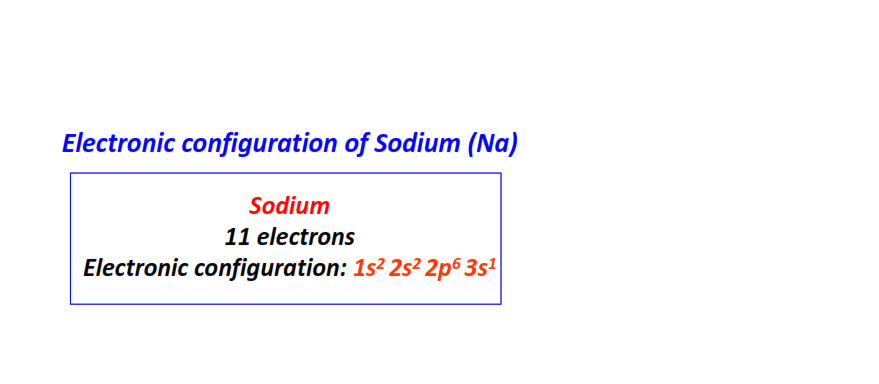
| 11 | Sodium (Na) | 1s22s22p63s1
|
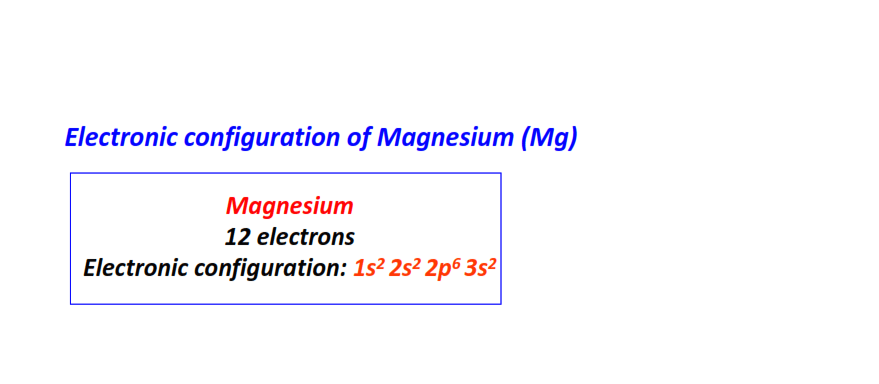
| 12 | Magnesium (Mg) | 1s22s22p63s2
|
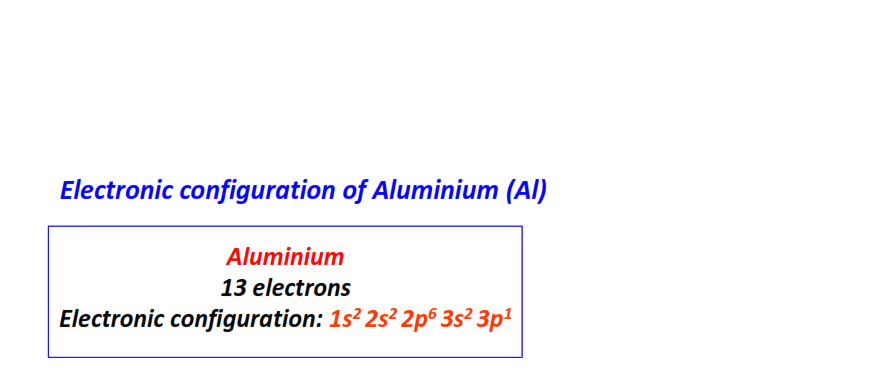
| 13 | Aluminum (Al) | 1s22s22p63s23p1
|
| 14 | Silicon (Si) | 1s22s22p63s23p2
|
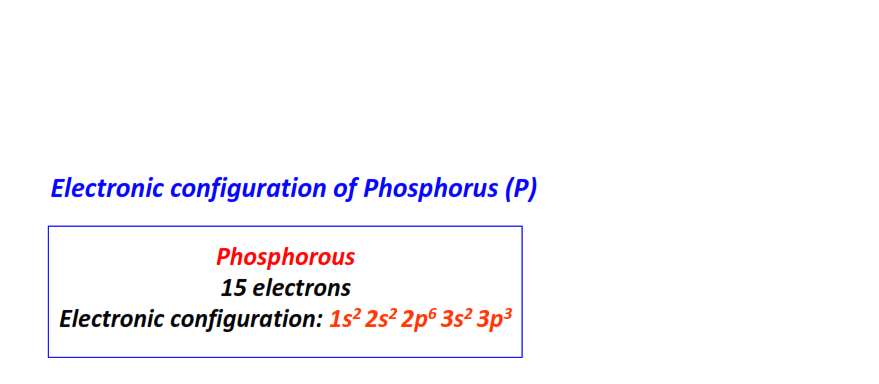
| 15 | Phosphorous (P) | 1s22s22p63s23p3
|
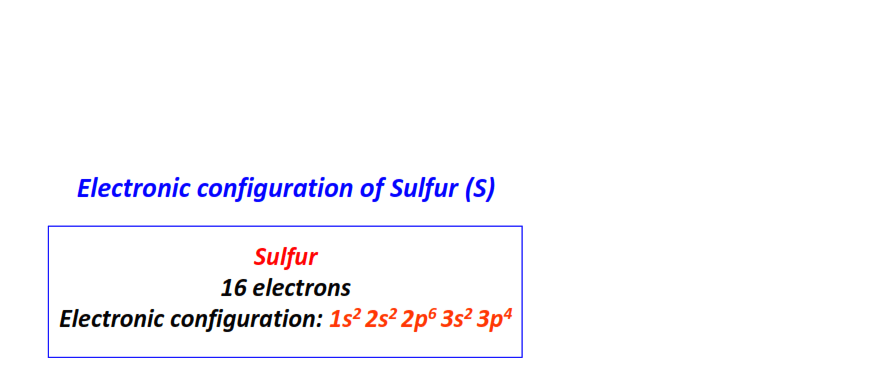
| 16 | Sulfur (S) | 1s22s22p63s23p4
|
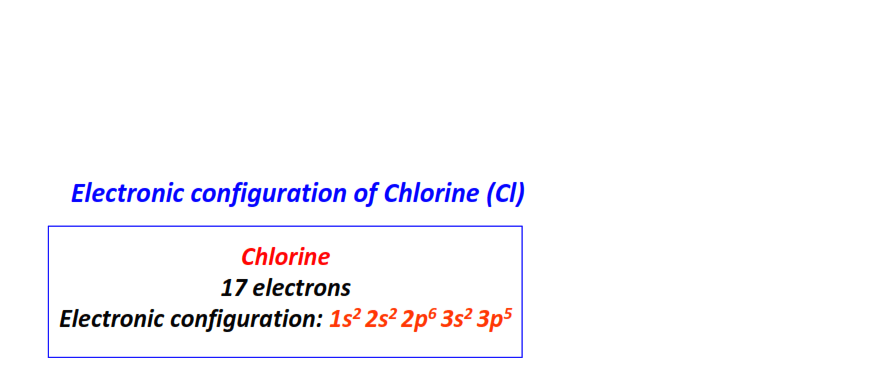
| 17 | Chlorine (Cl) | 1s22s22p63s23p5
|
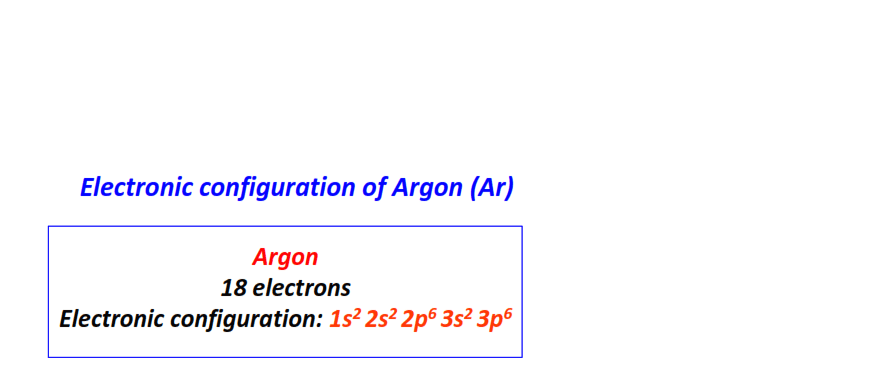
| 18 | Argon (Ar) | 1s22s22p63s23p6
|
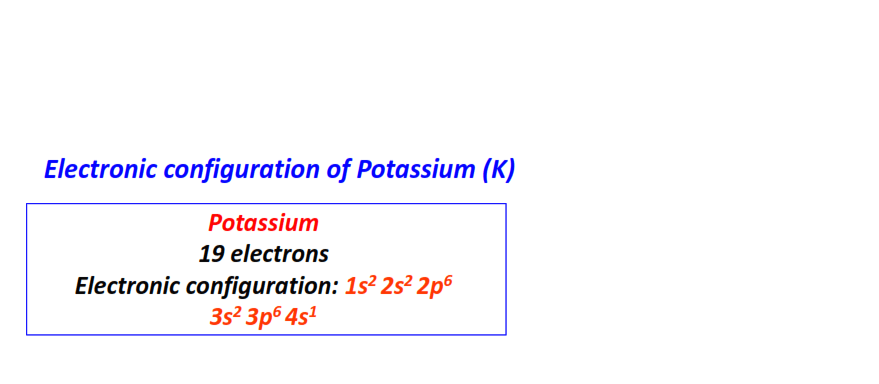
| 19 | Potassium (K) | 1s22s22p63s23p64s1
|
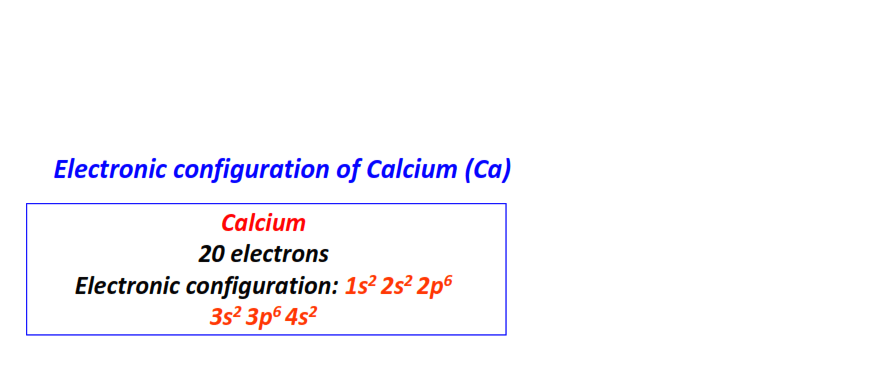
| 20 | Calcium (Ca) | 1s22s22p63s23p64s2
|
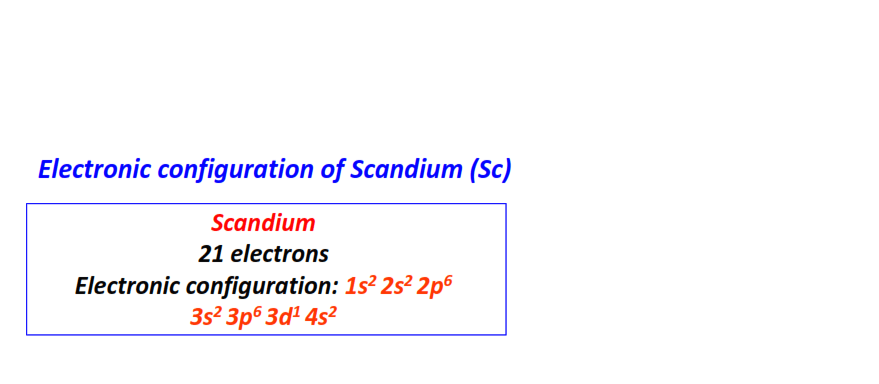
| 21 | Scandium (Sc) | 1s22s22p63s23p63d14s2
|
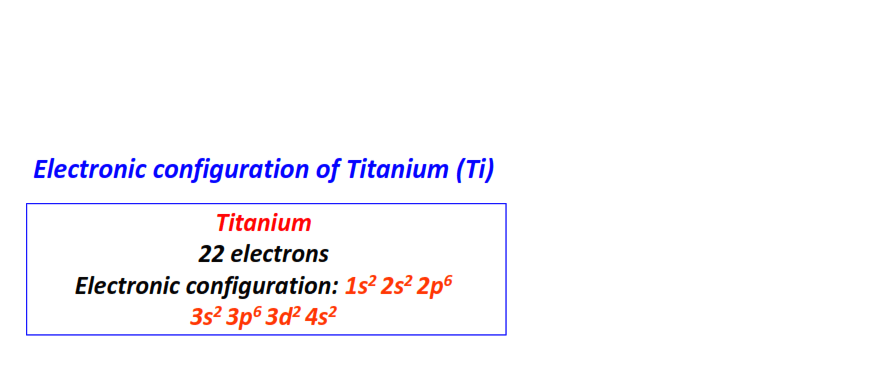
| 22 | Titanium (Ti) | 1s22s22p63s23p63d24s2
|
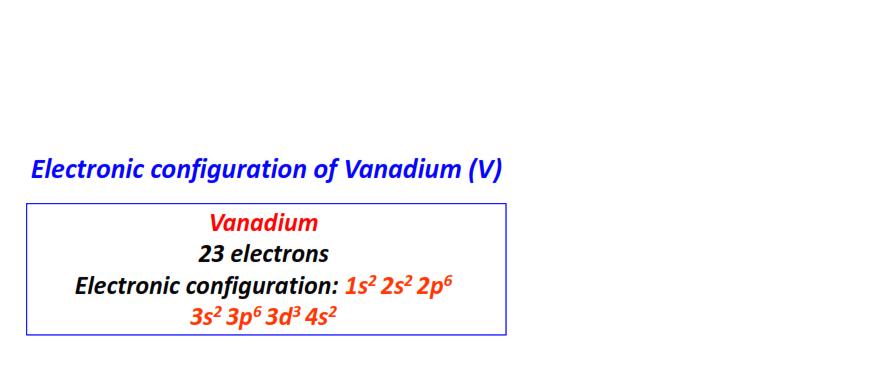
| 23 | Vanadium (V) | 1s22s22p63s23p63d34s2
|
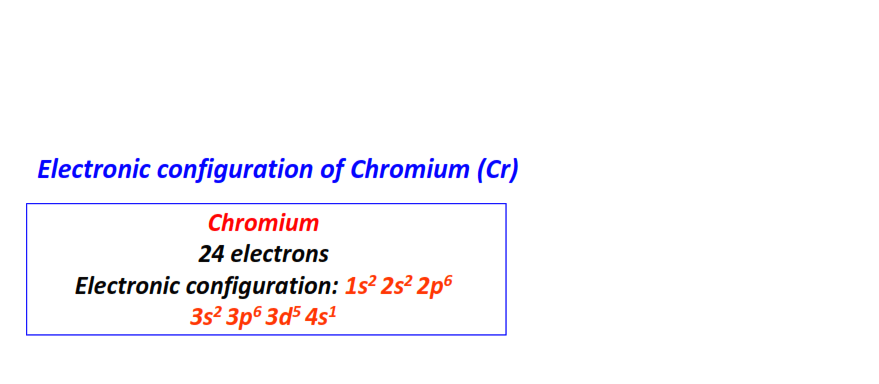
| 24 | Chromium (Cr) | 1s22s22p63s23p63d54s1
|
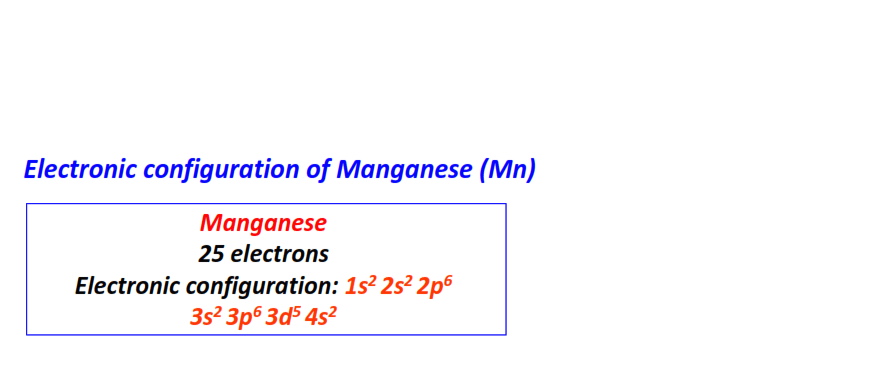
| 25 | Manganese (Mn) | 1s22s22p63s23p63d54s2
|
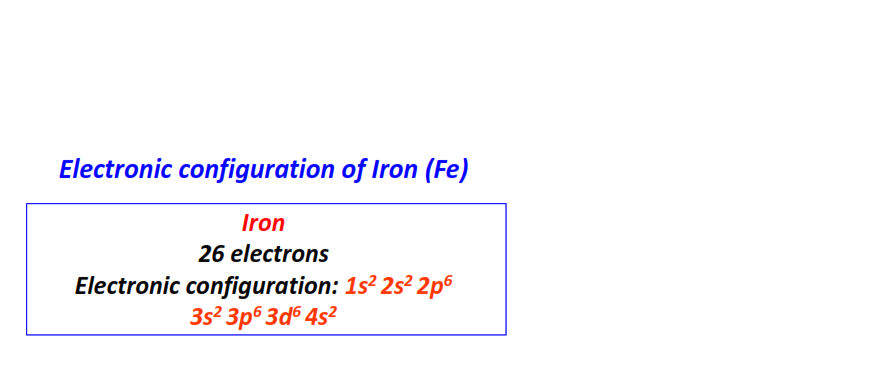
| 26 | Iron (Fe) | 1s22s22p63s23p63d64s2
|
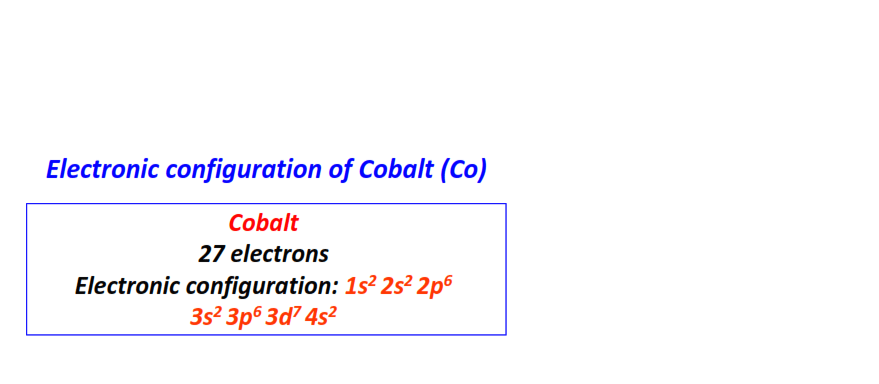
| 27 | Cobalt (Co) | 1s22s22p63s23p63d74s2
|
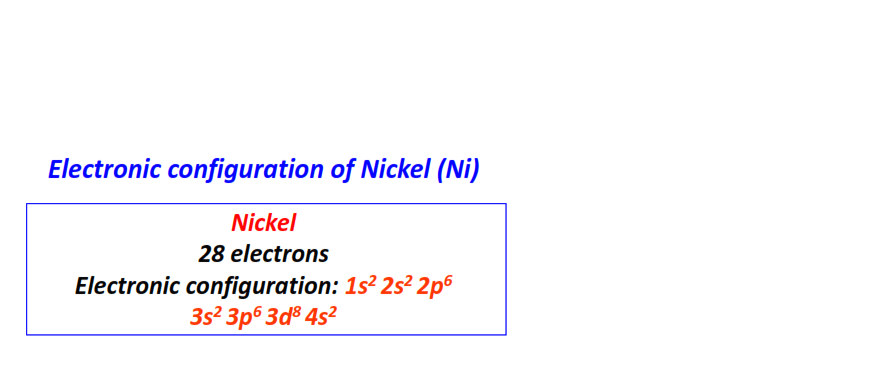
| 28 | Nickel (Ni) | 1s22s22p63s23p63d84s2
|
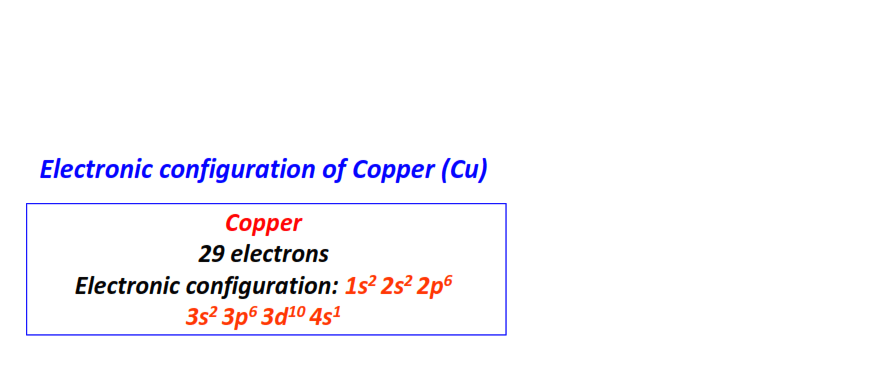
| 29 | Copper (Cu) | 1s22s22p63s23p63d104s1
|
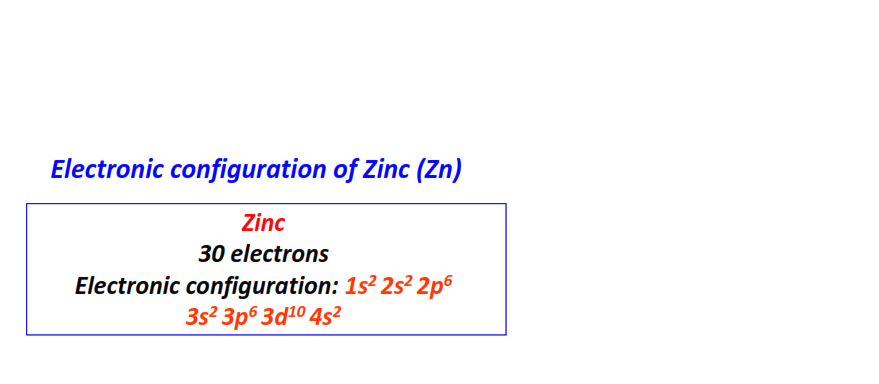
| 30 | Zinc (Zn) | 1s22s22p63s23p63d104s2
|
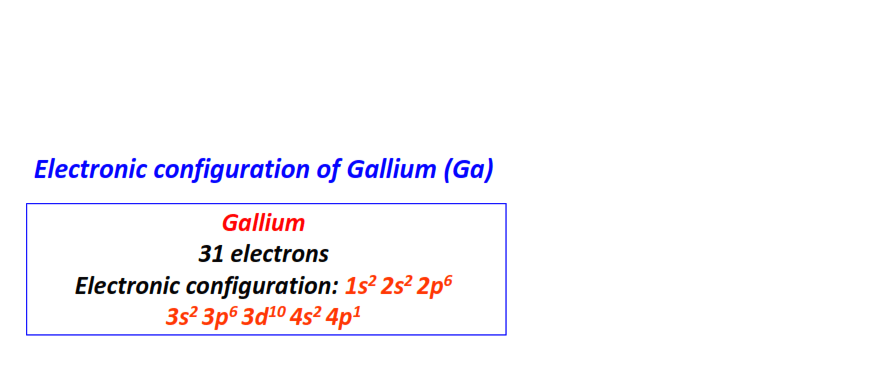
| 31 | Gallium (Ga) | 1s22s22p63s23p63d104s24p1
|
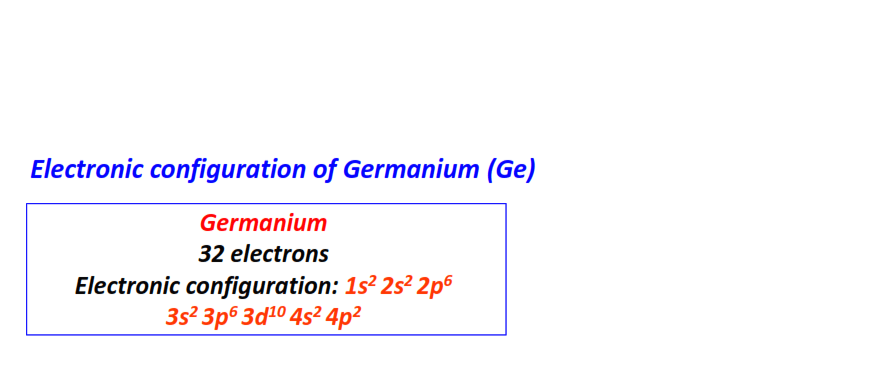
| 32 | Germanium (Ge) | 1s22s22p63s23p63d104s24p2
|
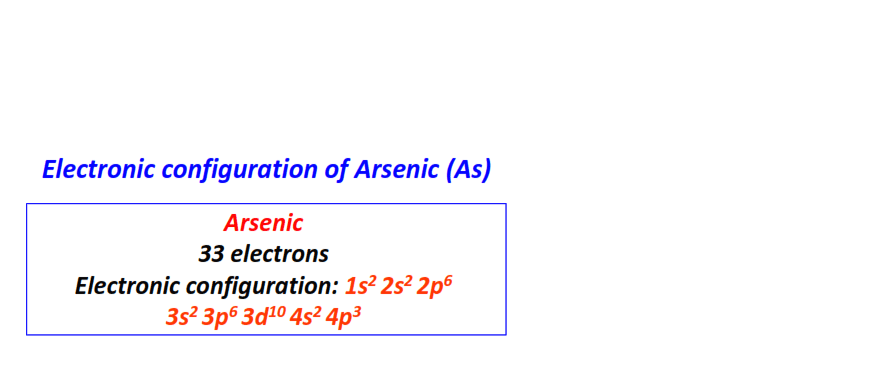
| 33 | Arsenic (As) | 1s22s22p63s23p63d104s24p3
|
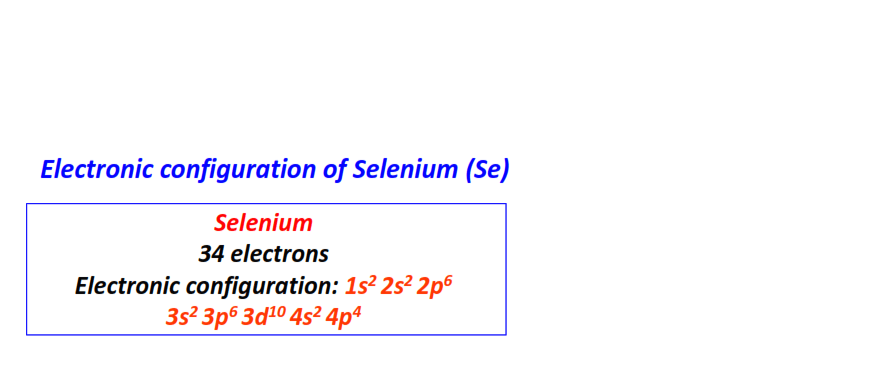
| 34 | Selenium (Se) | 1s22s22p63s23p63d104s24p4
|
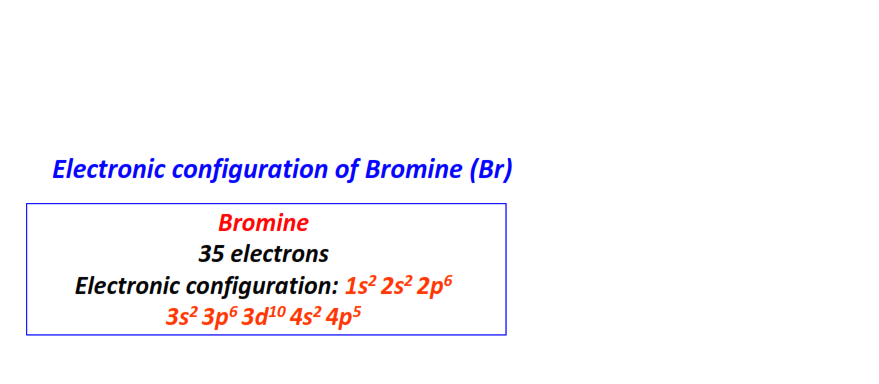
| 35 | Bromine (Br) | 1s22s22p63s23p63d104s24p5
|
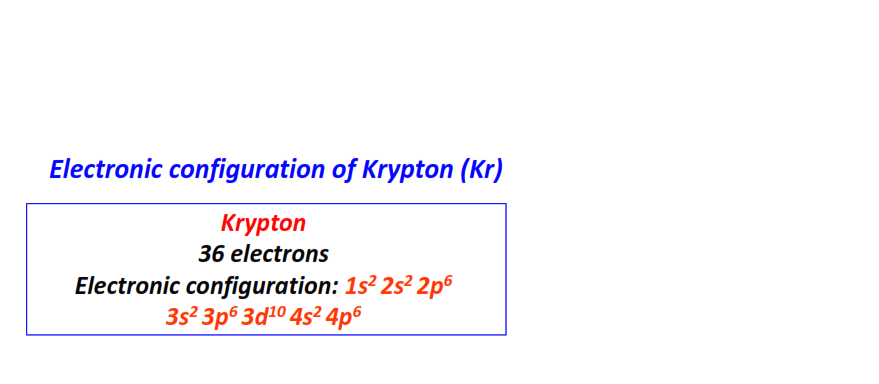
| 36 | Krypton (Kr) | 1s22s22p63s23p63d104s24p6
|
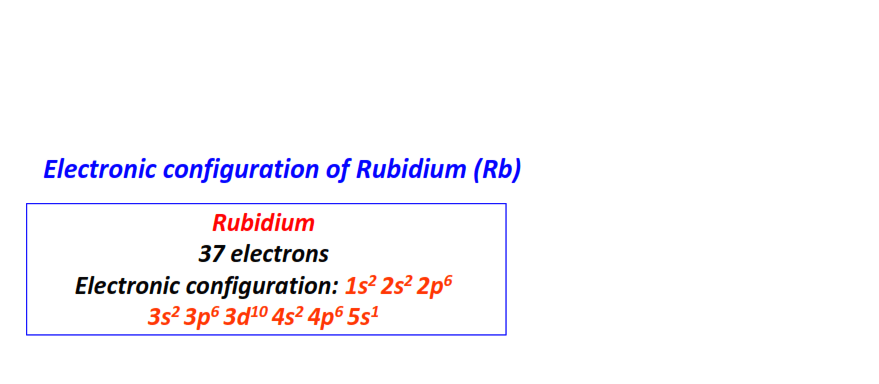
| 37 | Rubidium (Rb) | 1s22s22p63s23p63d104s24p65s1
|
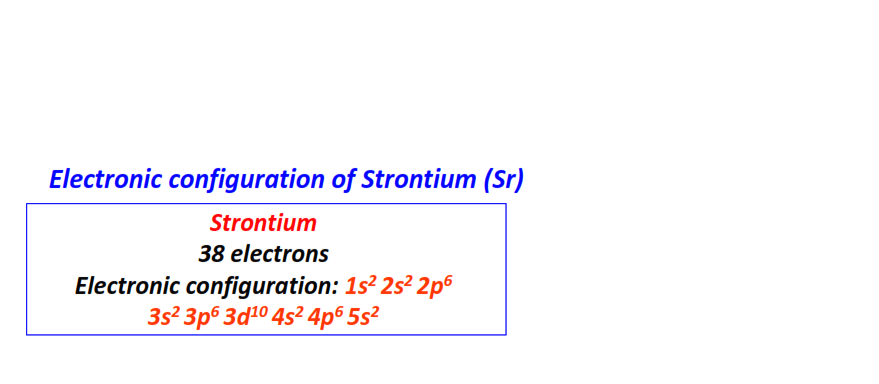
| 38 | Strontium (Sr) | 1s22s22p63s23p63d104s24p65s2
|
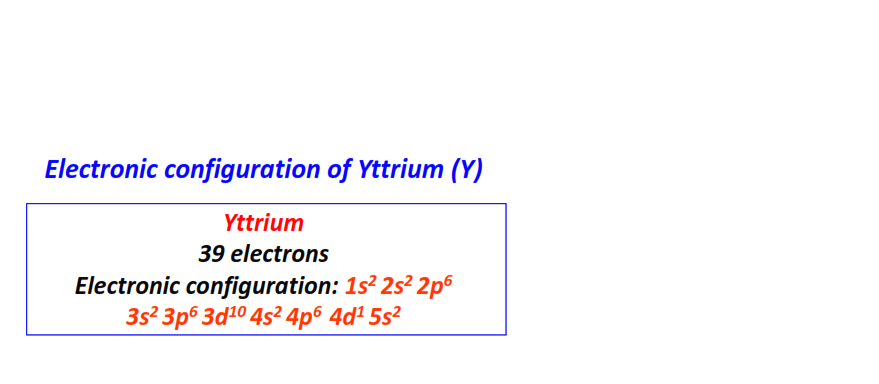
| 39 | Yttrium (Y) | 1s22s22p63s23p63d104s24p64d15s2
|
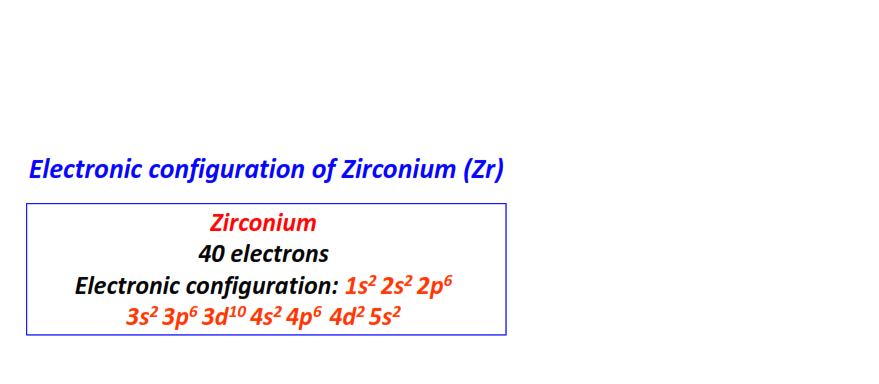
| 40 | Zirconium (Zr) | 1s22s22p63s23p63d104s24p64d25s2
|
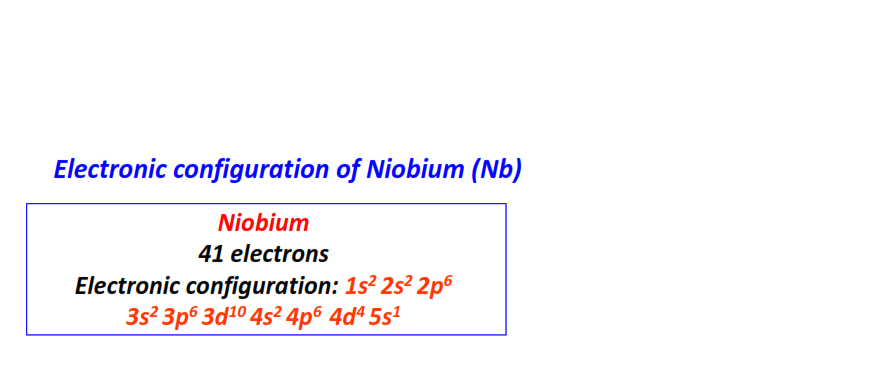
| 41 | Niobium (Nb) | 1s22s22p63s23p63d104s24p64d45s1
|
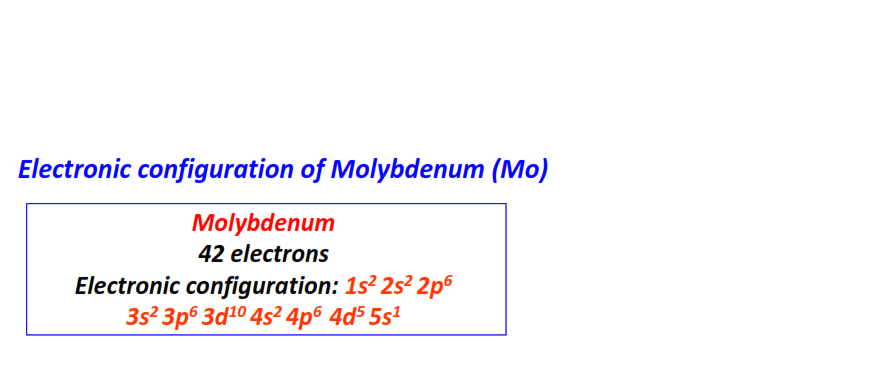
| 42 | Molybdenum (Mb) | 1s22s22p63s23p63d104s24p64d55s1
|
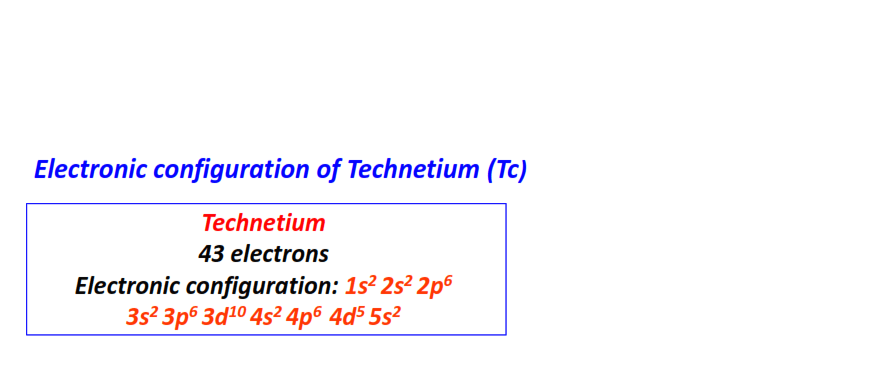
| 43 | Technetium (Tc) | 1s22s22p63s23p63d104s24p64d55s2
|
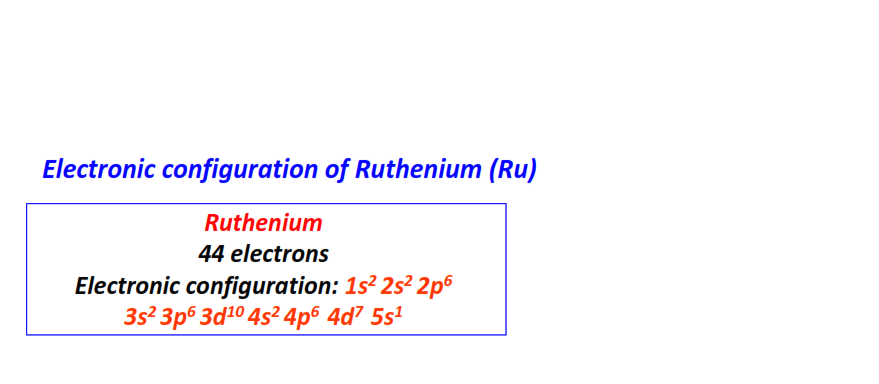
| 44 | Ruthenium (Ru) | 1s22s22p63s23p63d104s24p64d75s1
|
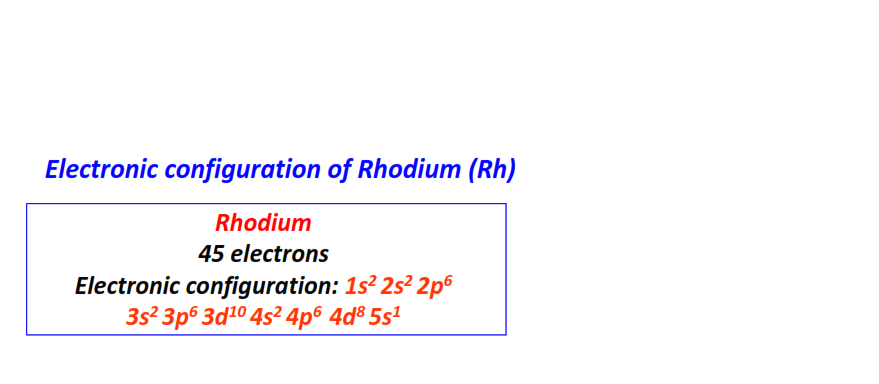
| 45 | Rhodium (Rh) | 1s22s22p63s23p63d104s24p64d85s1
|
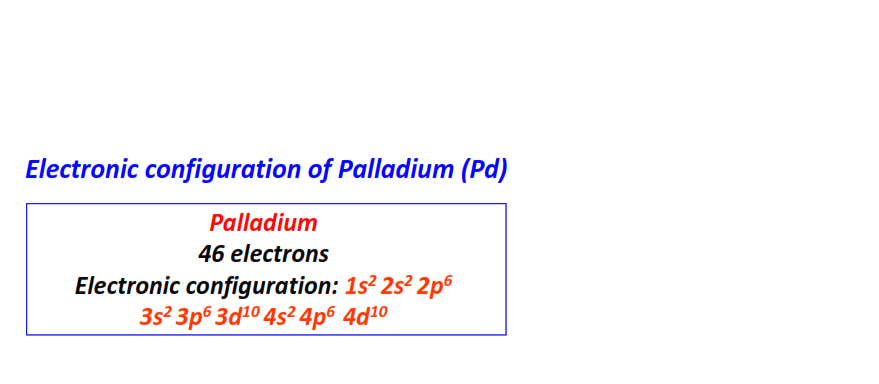
| 46 | Palladium (Pd) | 1s22s22p63s23p63d104s24p64d10
|
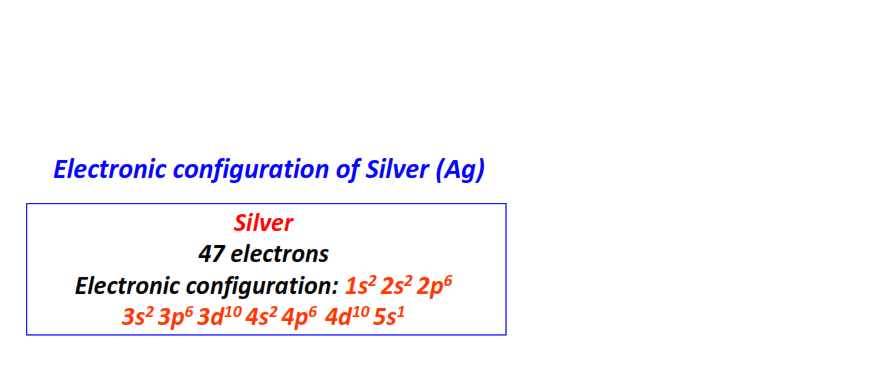
| 47 | Silver (Ag) | 1s22s22p63s23p63d104s24p64d105s1
|
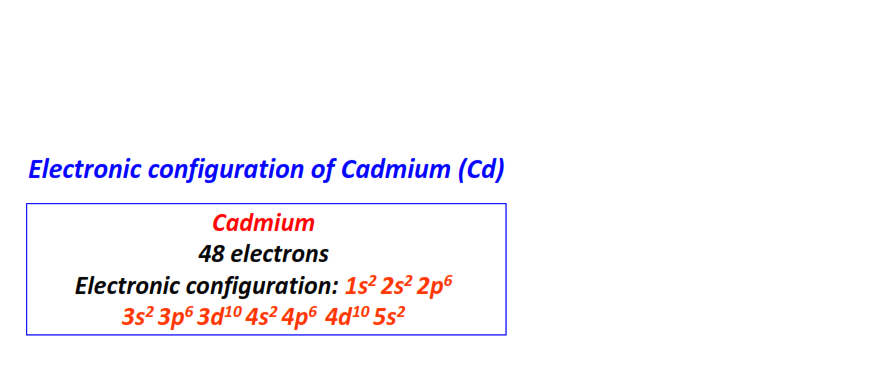
| 48 | Cadmium (Cd) | 1s22s22p63s23p63d104s24p64d105s2
|
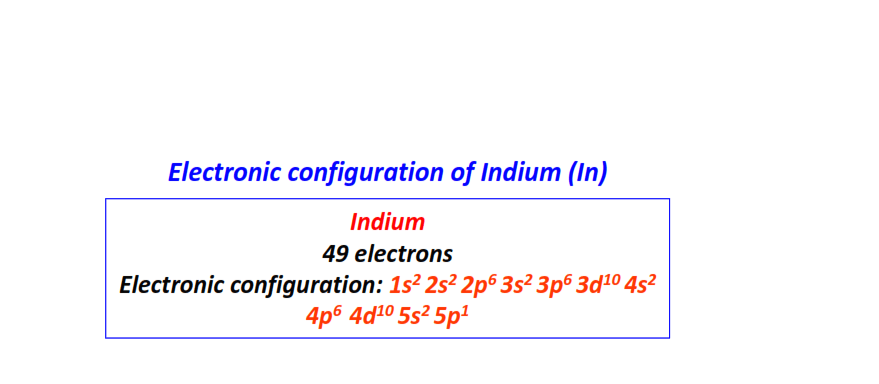
| 49 | Indium (In) | 1s22s22p63s23p63d104s24p64d105s25p1
|
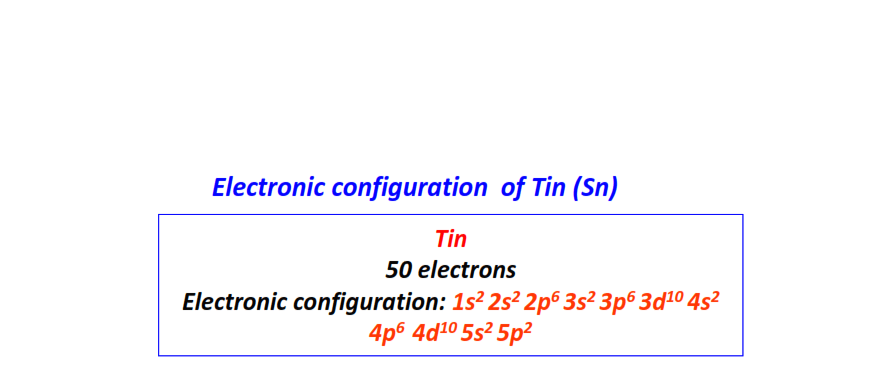
| 50 | Tin (Sn) | 1s22s22p63s23p63d104s24p64d105s25p2
|
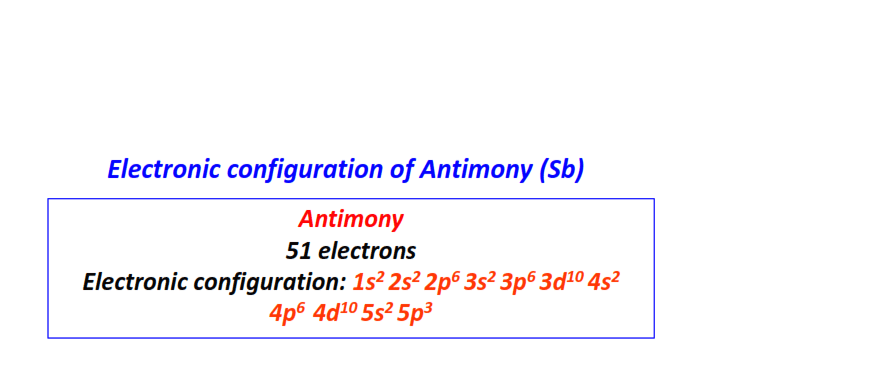
| 51 | Antimony (Sb) | 1s22s22p63s23p63d104s24p64d105s25p3
|
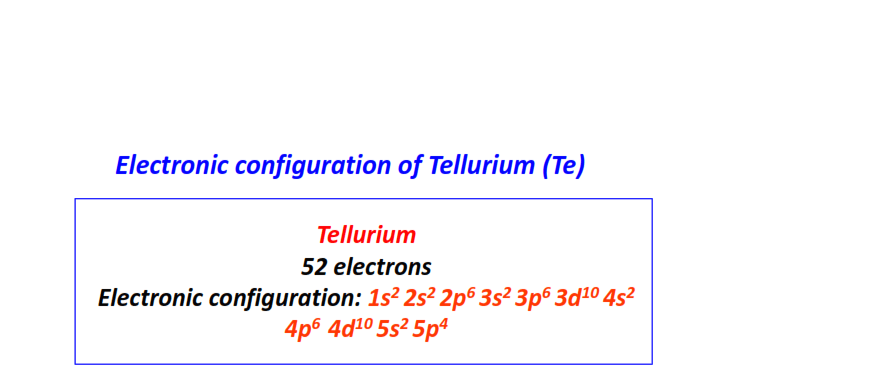
| 52 | Tellurium (Te) | 1s22s22p63s23p63d104s24p64d105s25p4
|
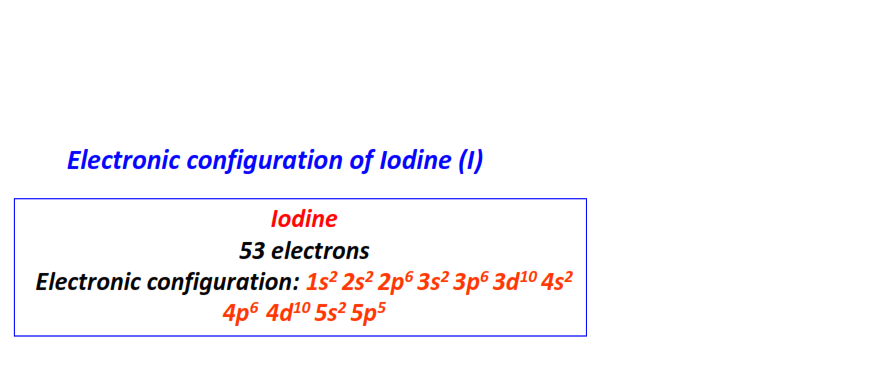
| 53 | Iodine (I) | 1s22s22p63s23p63d104s24p64d105s25p5
|
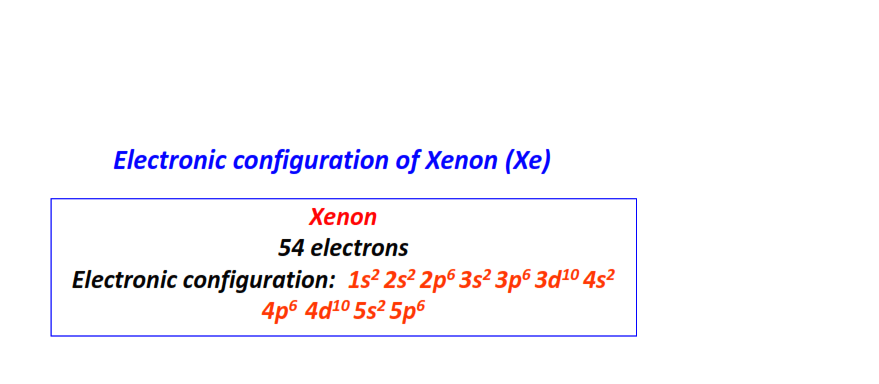
| 54 | Xenon (Xe) | 1s22s22p63s23p63d104s24p64d105s25p6
|
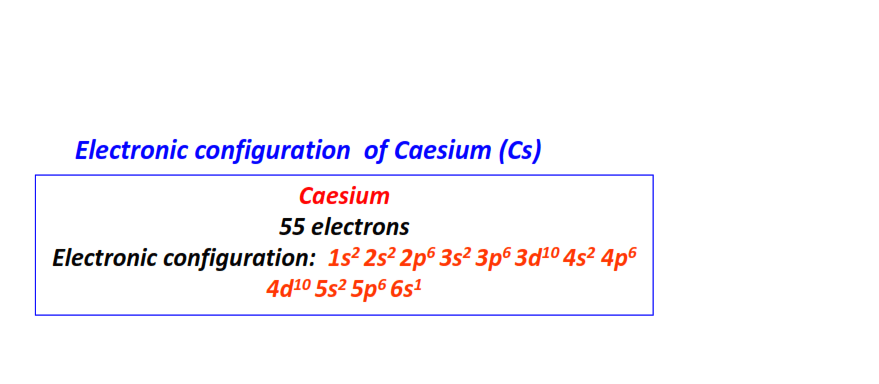
| 55 | Cesium (Cs) | 1s22s22p63s23p63d104s24p64d105s25p66s1
|
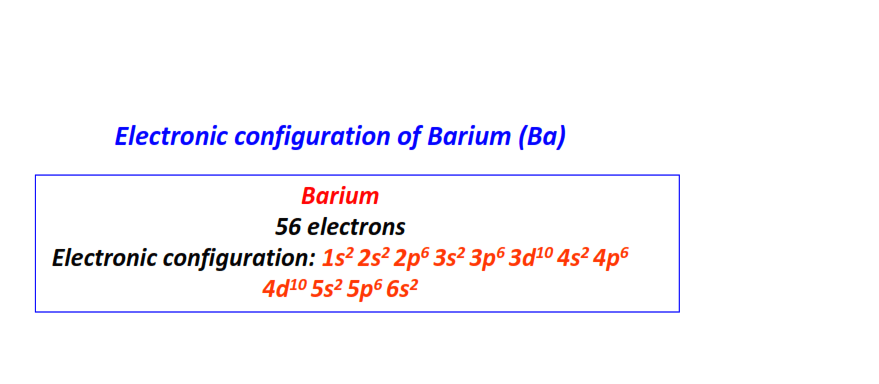
| 56 | Barium (Ba) | 1s22s22p63s23p63d104s24p64d105s25p66s2
|
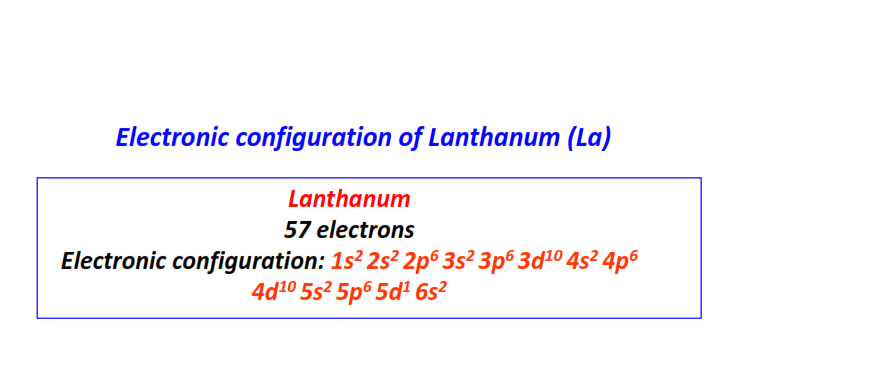
| 57 | Lanthanum (La) | 1s22s22p63s23p63d104s24p64d105s25p65d16s2
|
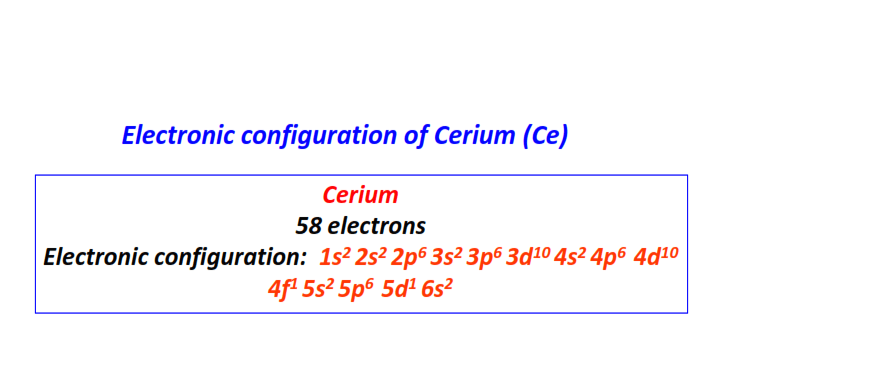
| 58 | Cerium (Ce) | 1s22s22p63s23p63d104s24p64d104f15s25p65d16s2
|
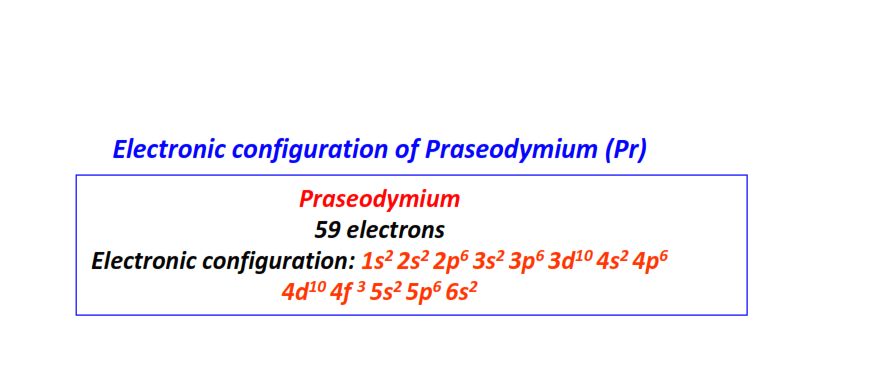
| 59 | Praseodymium (Pr) | 1s22s22p63s23p63d104s24p64d104f35s25p66s2
|
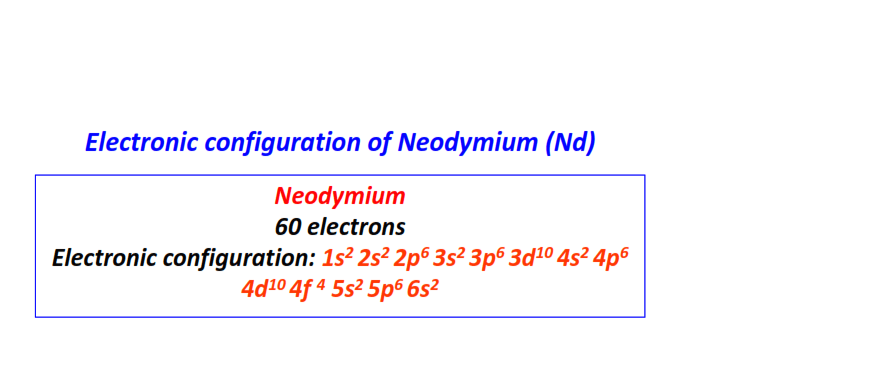
| 60 | Neodymium (Nd) | 1s22s22p63s23p63d104s24p64d104f45s25p66s2
|
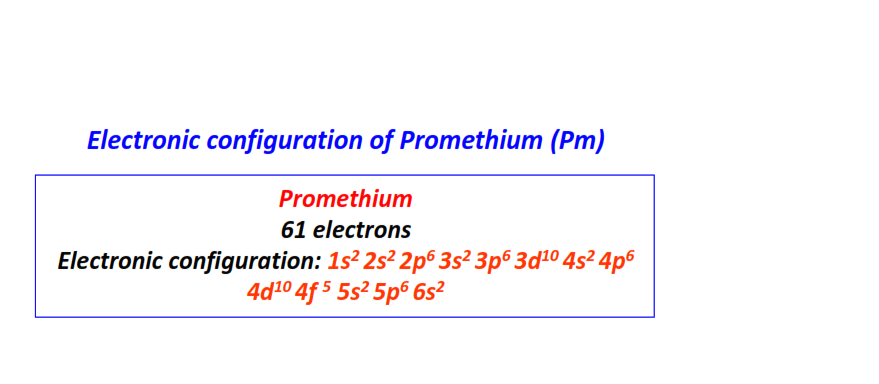
| 61 | Promethium (Pm) | 1s22s22p63s23p63d104s24p64d104f55s25p66s2
|
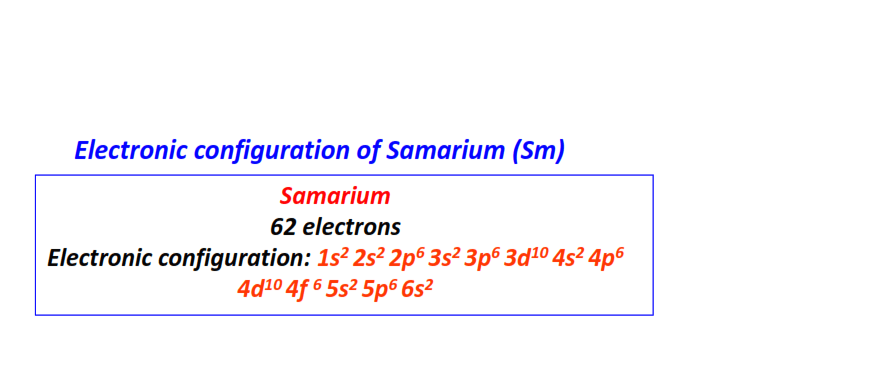
| 62 | Samarium (Sm) | 1s22s22p63s23p63d104s24p64d104f65s25p66s2
|
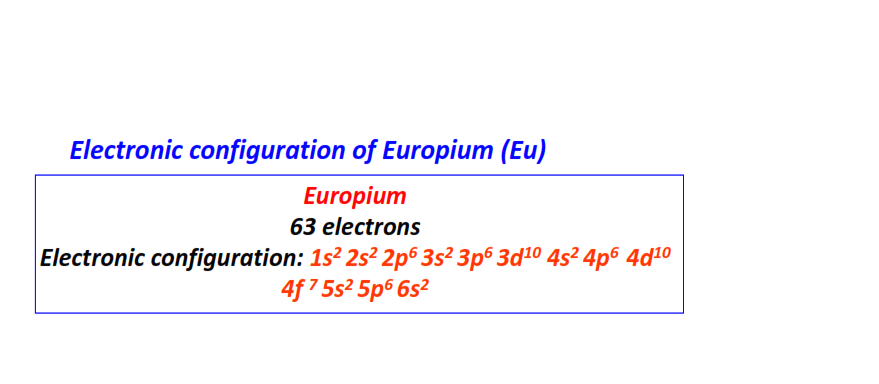
| 63 | Europium (Eu) | 1s22s22p63s23p63d104s24p64d104f75s25p66s2
|
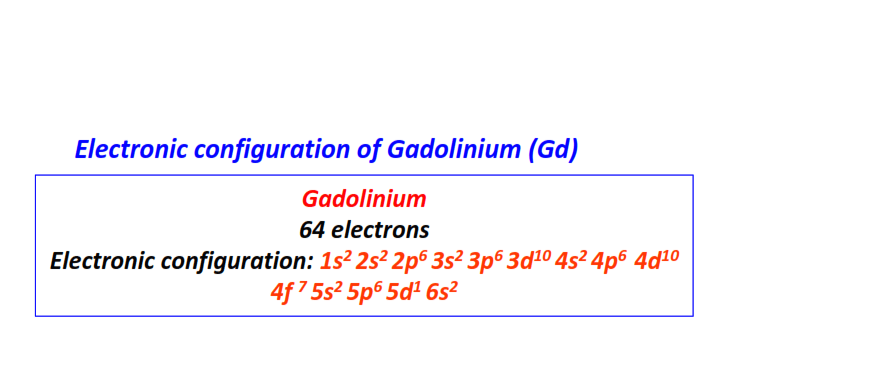
| 64 | Gadolinium (Gd) | 1s22s22p63s23p63d104s24p64d104f75s25p65d16s2
|
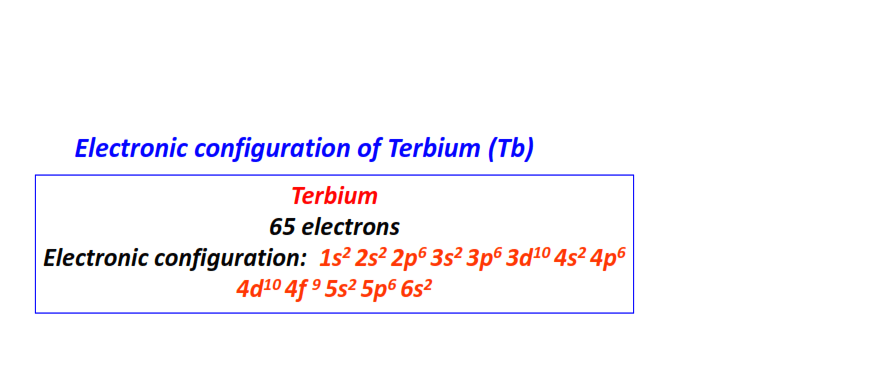
| 65 | Terbium (Tb) | 1s22s22p63s23p63d104s24p64d104f95s25p66s2
|
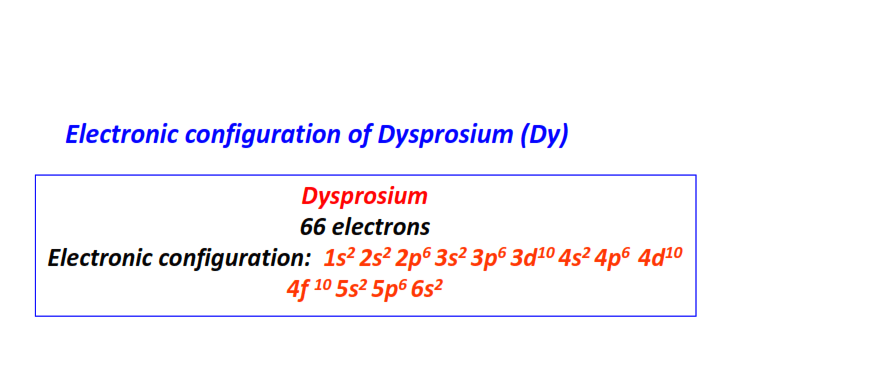
| 66 | Dysprosium (Dy) | 1s22s22p63s23p63d104s24p64d104f105s25p66s2
|
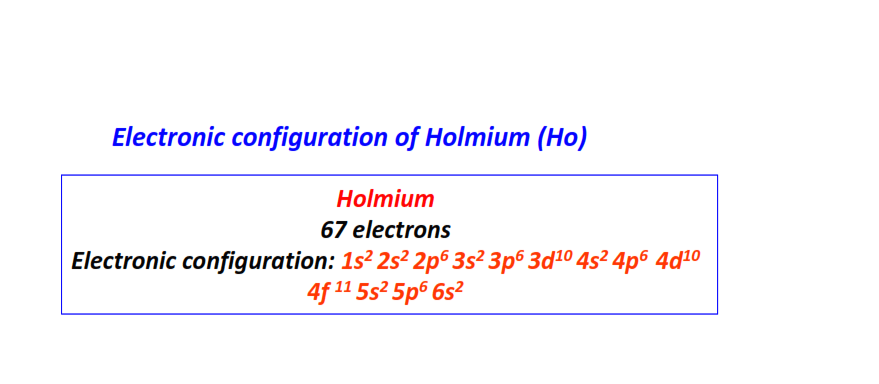
| 67 | Holmium (Ho) | 1s22s22p63s23p63d104s24p64d104f115s25p66s2
|
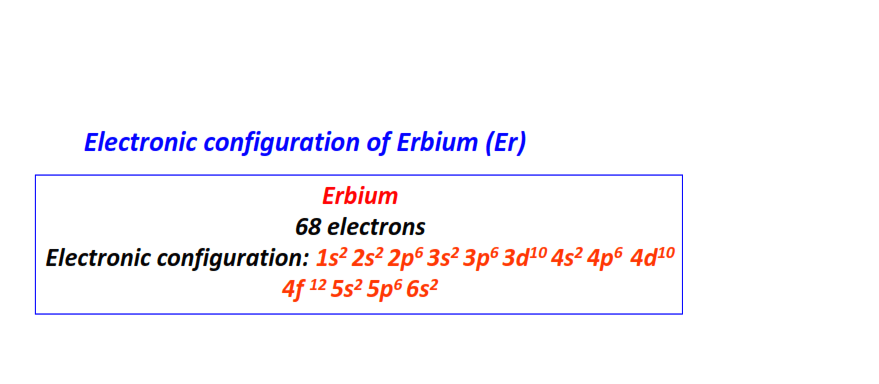
| 68 | Erbium (Er) | 1s22s22p63s23p63d104s24p64d104f125s25p66s2
|
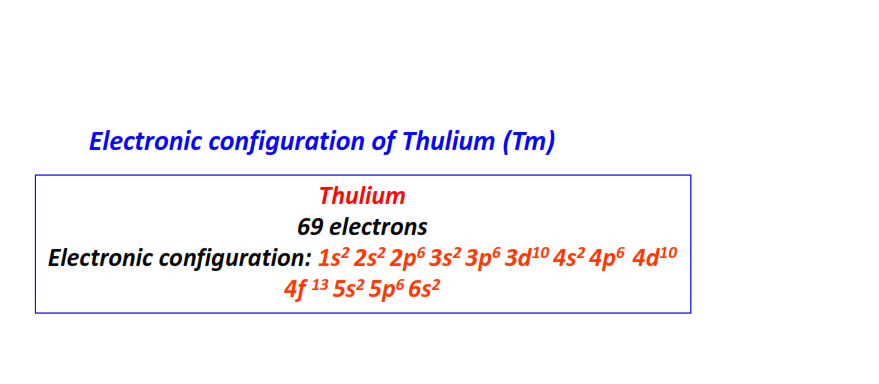
| 69 | Thulium (Tm) | 1s22s22p63s23p63d104s24p64d104f135s25p66s2
|
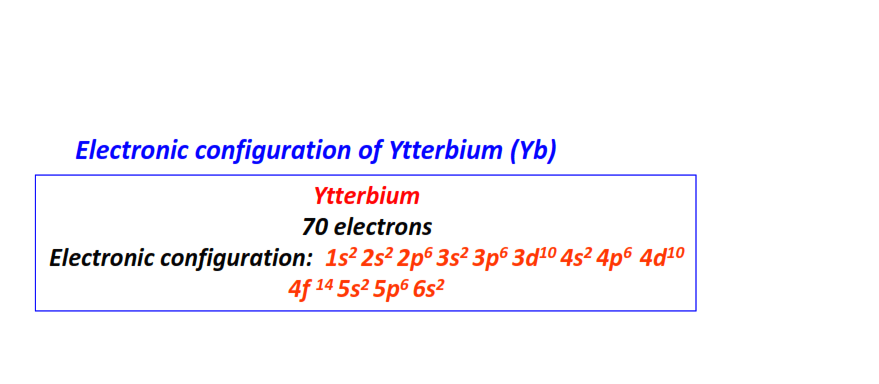
| 70 | Ytterbium (Yb) | 1s22s22p63s23p63d104s24p64d104f145s25p66s2
|
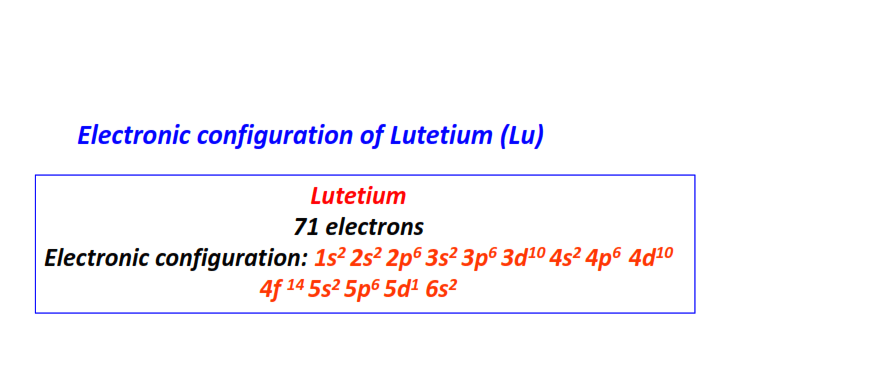
| 71 | Lutetium (Lu) | 1s22s22p63s23p63d104s24p64d104f145s25p65d16s2
|
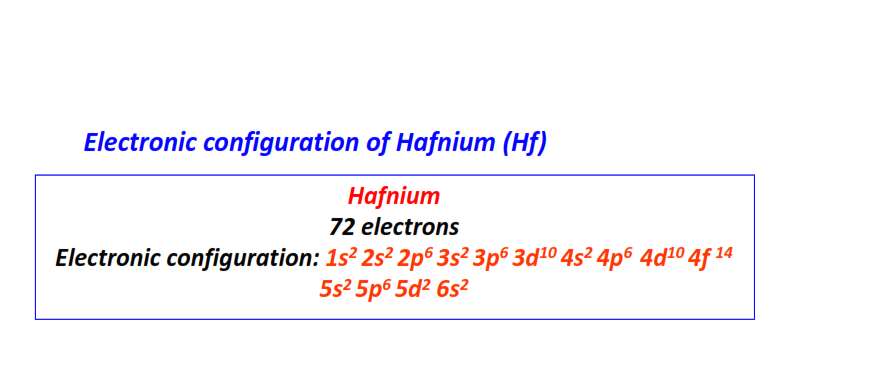
| 72 | Hafnium (Hf) | 1s22s22p63s23p63d104s24p64d104f145s25p65d26s2
|
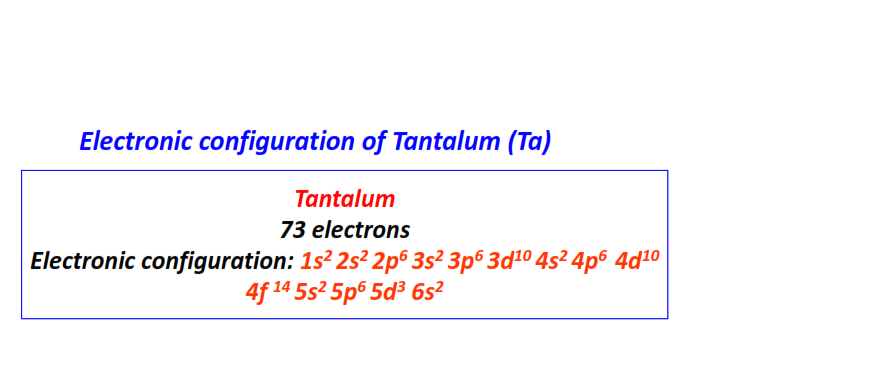
| 73 | Tantalum (Ta) | 1s22s22p63s23p63d104s24p64d104f145s25p65d36s2
|
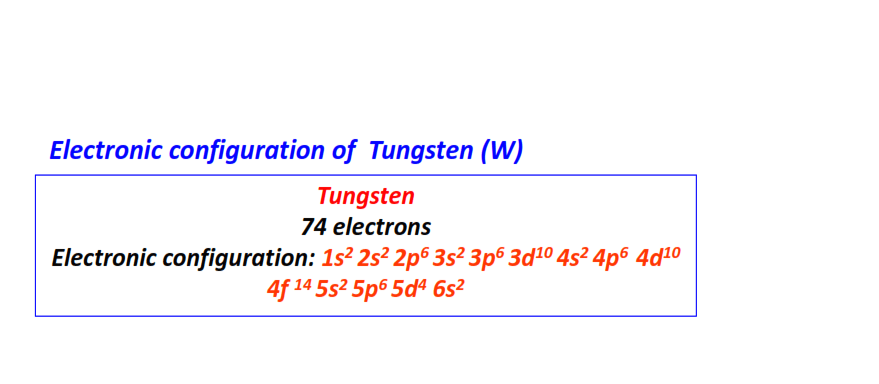
| 74 | Tungsten (W) | 1s22s22p63s23p63d104s24p64d104f145s25p65d46s2
|
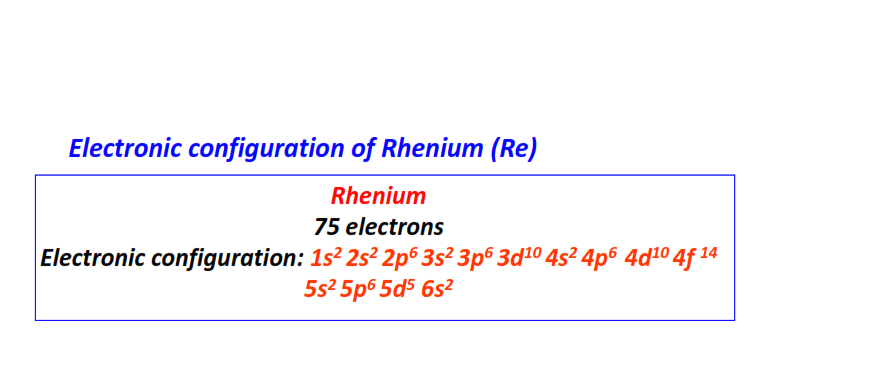
| 75 | Rhenium (Re) | 1s22s22p63s23p63d104s24p64d104f145s25p65d56s2
|
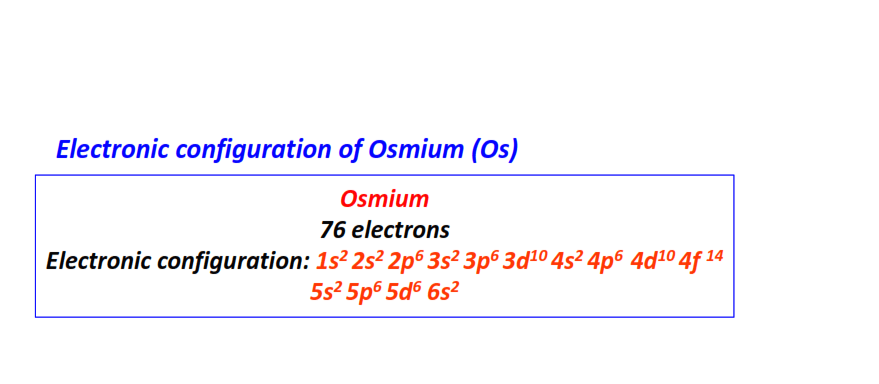
| 76 | Osmium (Os) | 1s22s22p63s23p63d104s24p64d104f145s25p65d66s2
|
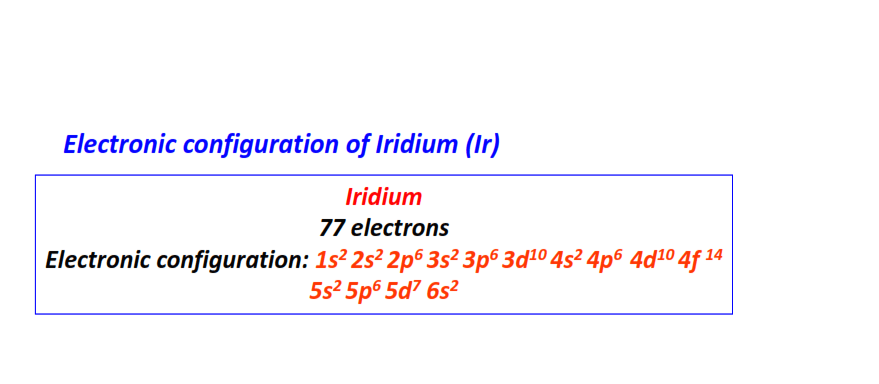
| 77 | Iridium (Ir) | 1s22s22p63s23p63d104s24p64d104f145s25p65d76s2
|
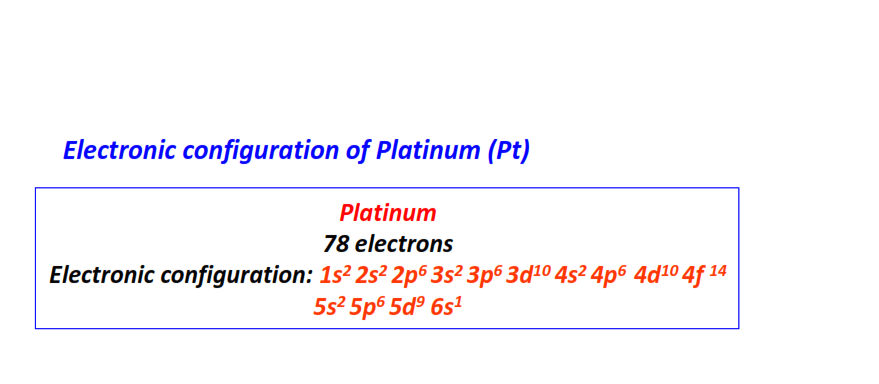
| 78 | Platinum (Pt) | 1s22s22p63s23p63d104s24p64d104f145s25p65d96s1
|
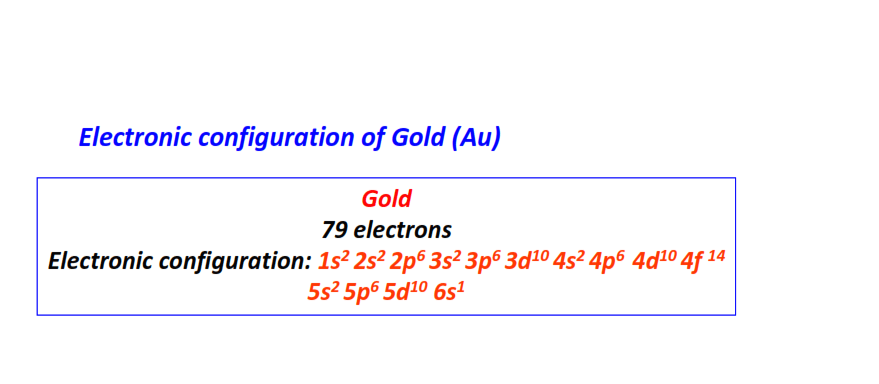
| 79 | Gold (Au) | 1s22s22p63s23p63d104s24p64d104f145s25p65d106s1
|
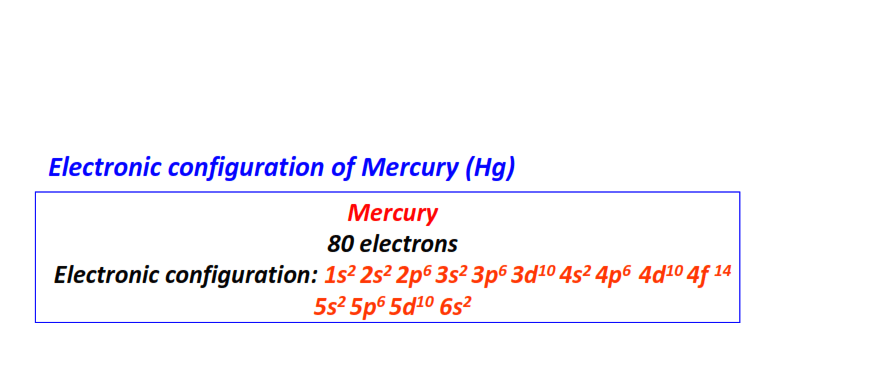
| 80 | Mercury (Hg) | 1s22s22p63s23p63d104s24p64d104f145s25p65d106s2
|
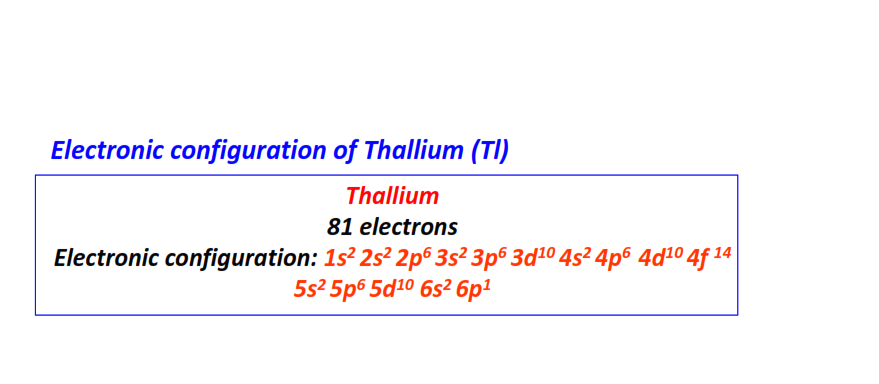
| 81 | Thallium (Tl) | 1s22s22p63s23p63d104s24p64d104f145s25p65d106s26p1
|
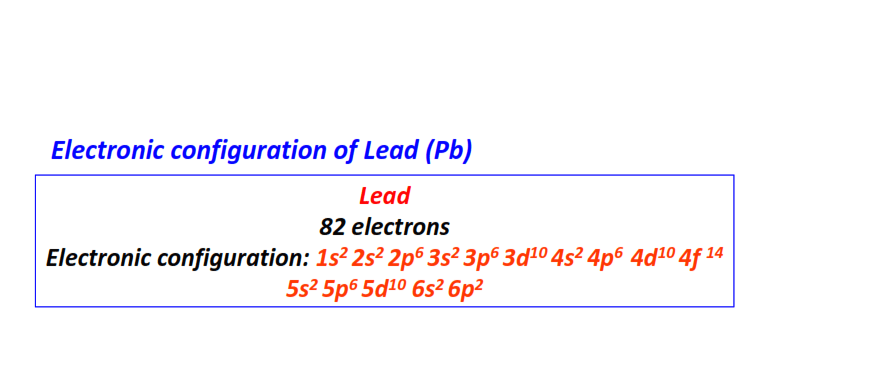
| 82 | Lead (Pb) | 1s22s22p63s23p63d104s24p64d104f145s25p65d106s26p2
|
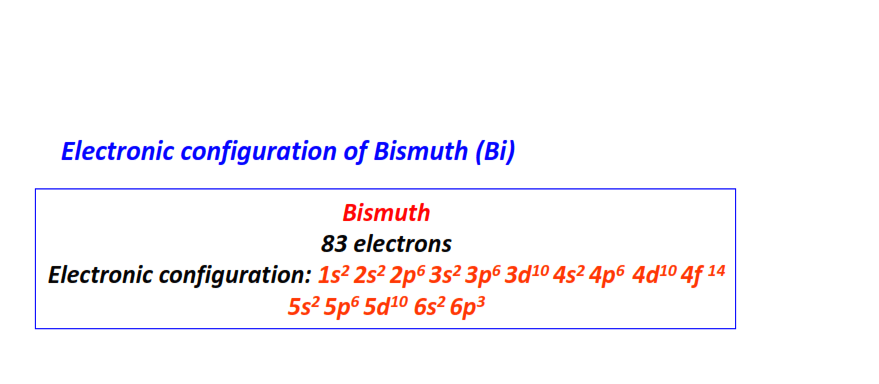
| 83 | Bismuth (Bi) | 1s22s22p63s23p63d104s24p64d104f145s25p65d106s26p3
|
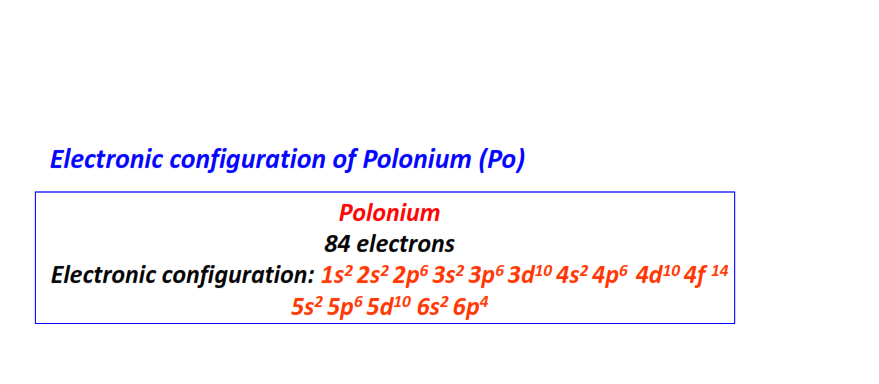
| 84 | Polonium (Po) | 1s22s22p63s23p63d104s24p64d104f145s25p65d106s26p4
|
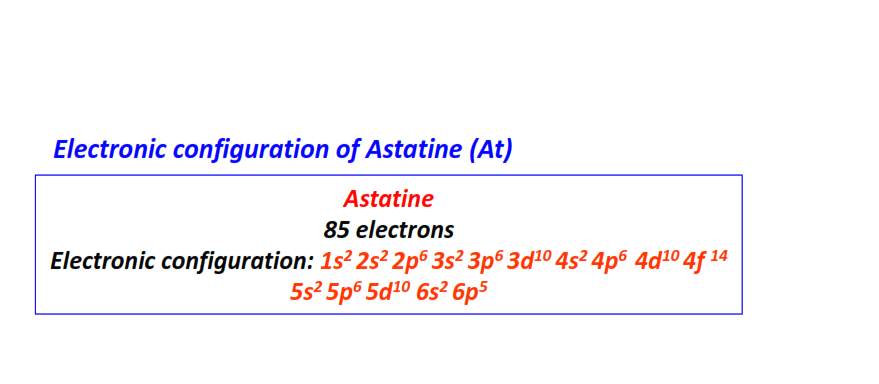
| 85 | Astatine (At) | 1s22s22p63s23p63d104s24p64d104f145s25p65d106s26p5
|
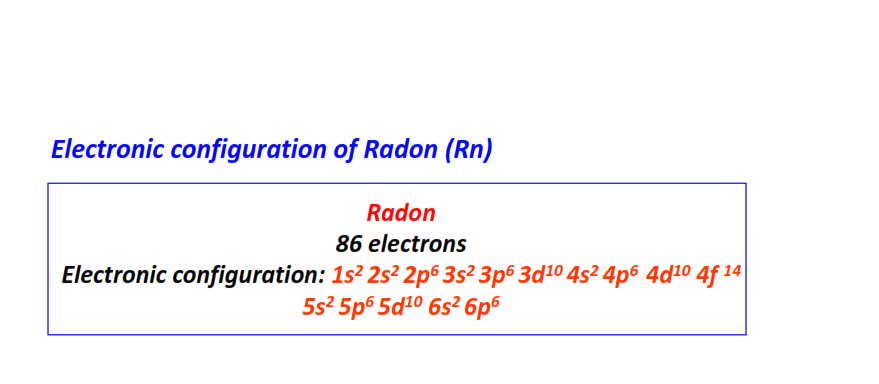
| 86 | Radon (Rn) | 1s22s22p63s23p63d104s24p64d104f145s25p65d106s26p6
|
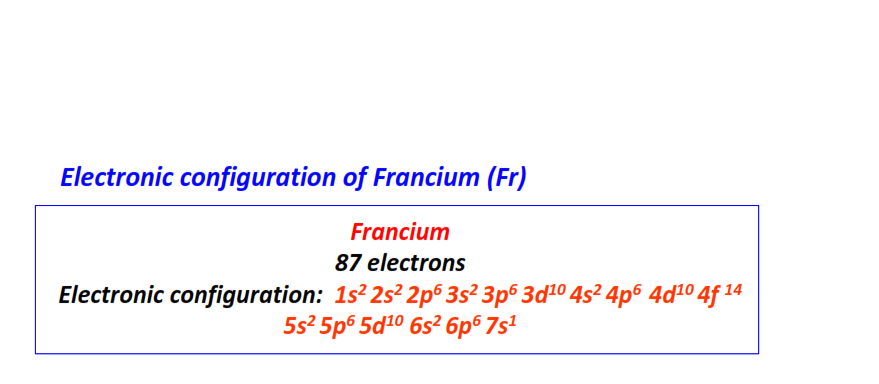
| 87 | Francium (Fr) | 1s22s22p63s23p63d104s24p64d104f145s25p65d106s26p67s1
|
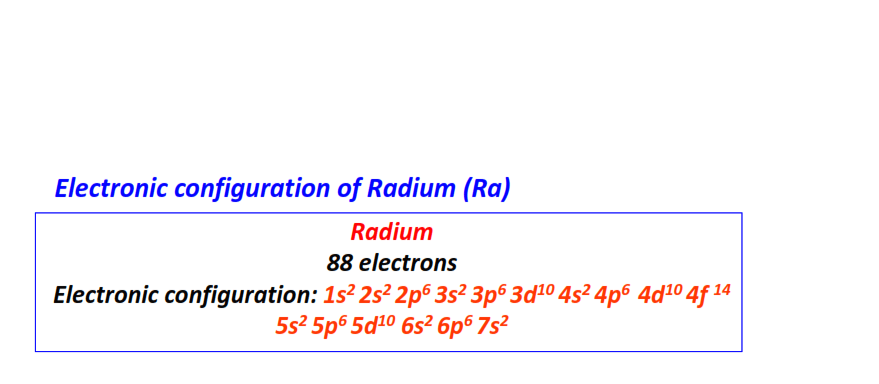
| 88 | Radium (Ra) | 1s22s22p63s23p63d104s24p64d104f145s25p65d106s26p67s2
|
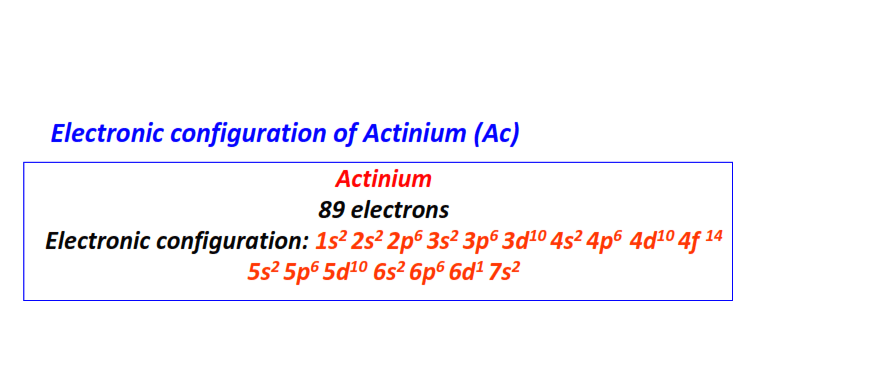
| 89 | Actinium (Ac) | 1s22s22p63s23p63d104s24p64d104f145s25p65d106s26p66d17s2
|
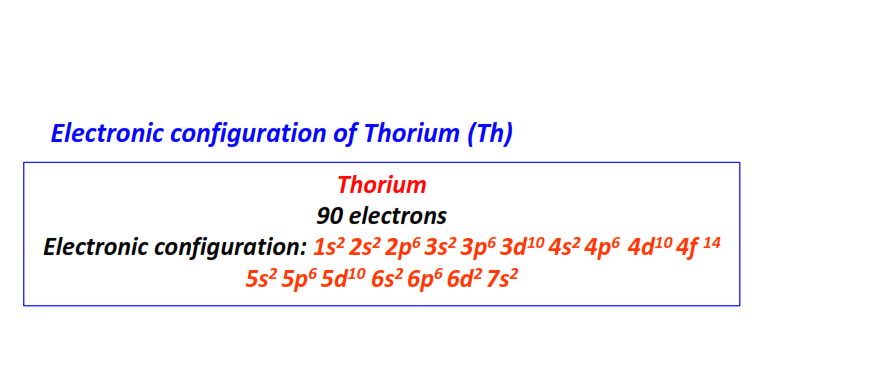
| 90 | Thorium (Th) | 1s22s22p63s23p63d104s24p64d104f145s25p65d106s26p66d27s2
|
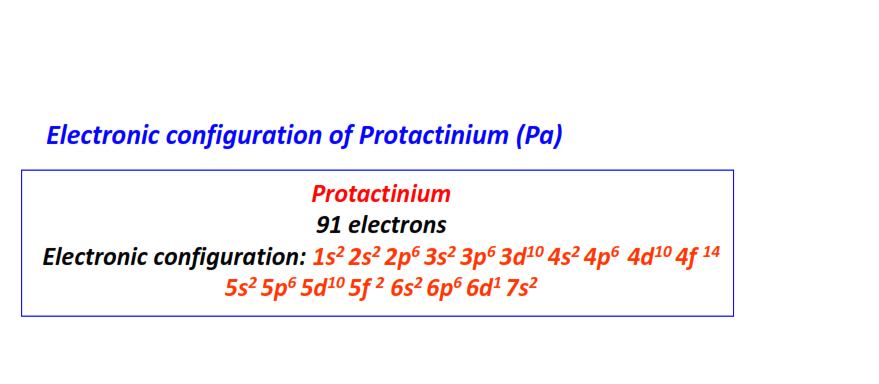
| 91 | Protactinium (Pa) | 1s22s22p63s23p63d104s24p64d104f145s25p65d105f26s26p66d17s2
|
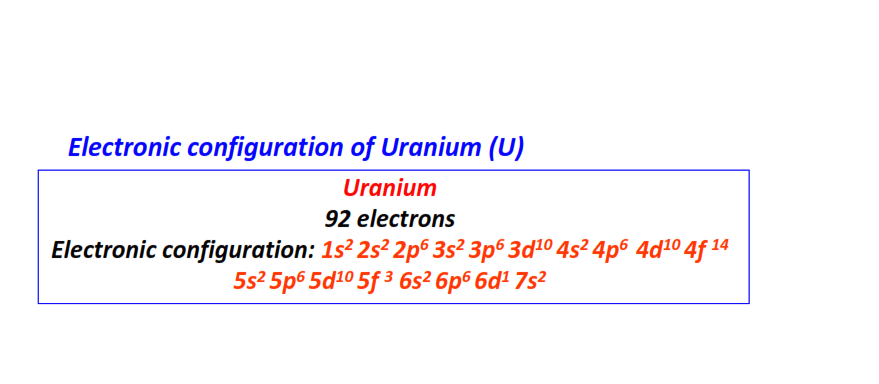
| 92 | Uranium (U) | 1s22s22p63s23p63d104s24p64d104f145s25p65d105f36s26p66d17s2
|
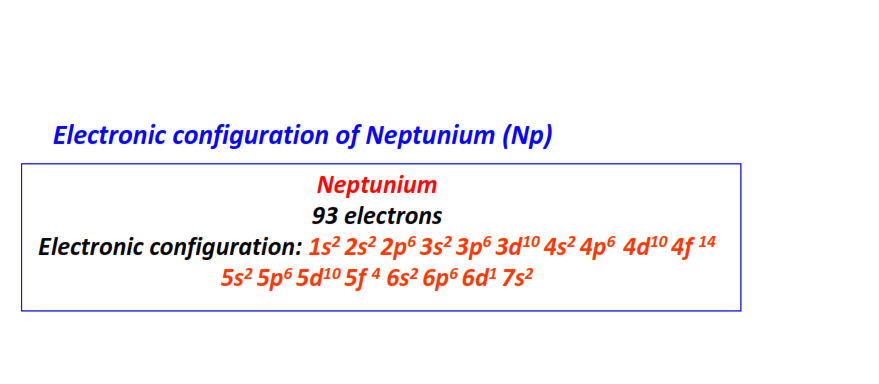
| 93 | Neptunium (Np) | 1s22s22p63s23p63d104s24p64d104f145s25p65d105f46s26p66d17s2
|
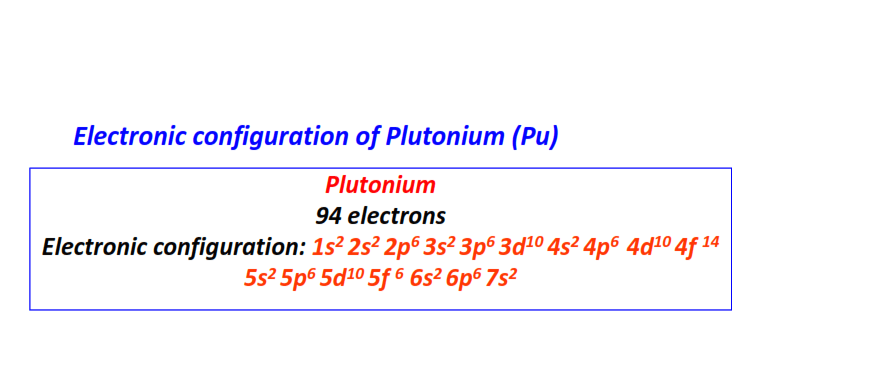
| 94 | Plutonium (Pu) | 1s22s22p63s23p63d104s24p64d104f145s25p65d105f66s26p67s2
|
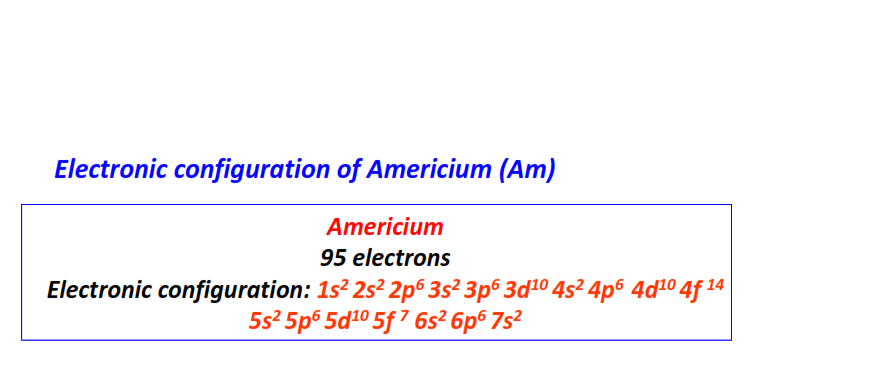
| 95 | Americium (Am) | 1s22s22p63s23p63d104s24p64d104f145s25p65d105f76s26p67s2
|
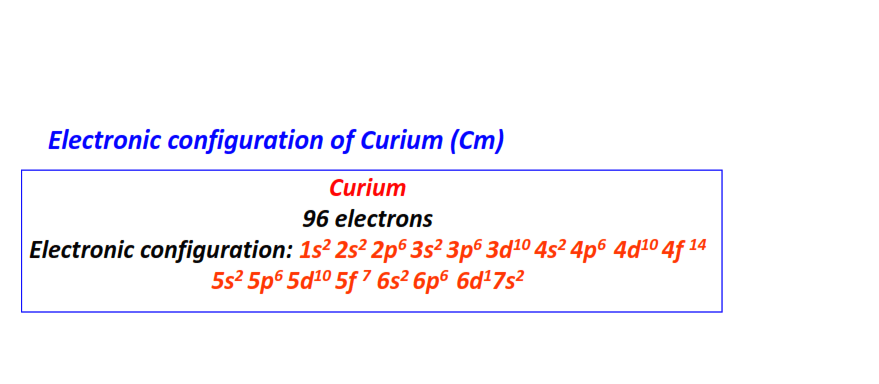
| 96 | Curium (Cm) | 1s22s22p63s23p63d104s24p64d104f145s25p65d105f76s26p66d17s2
|
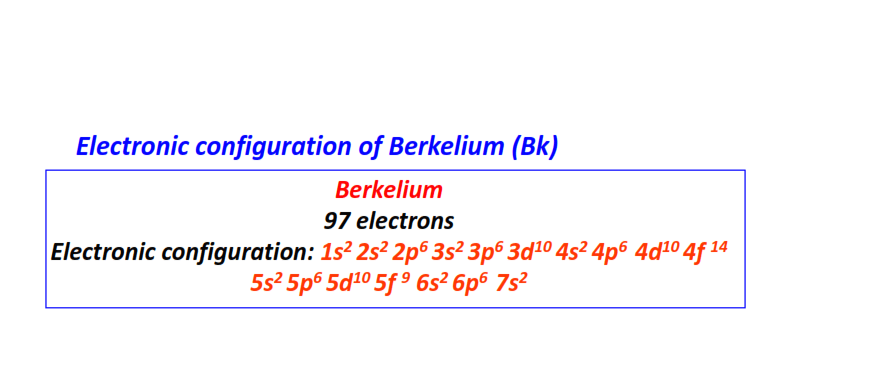
| 97 | Berkelium (Bk) | 1s22s22p63s23p63d104s24p64d104f145s25p65d105f96s26p67s2
|
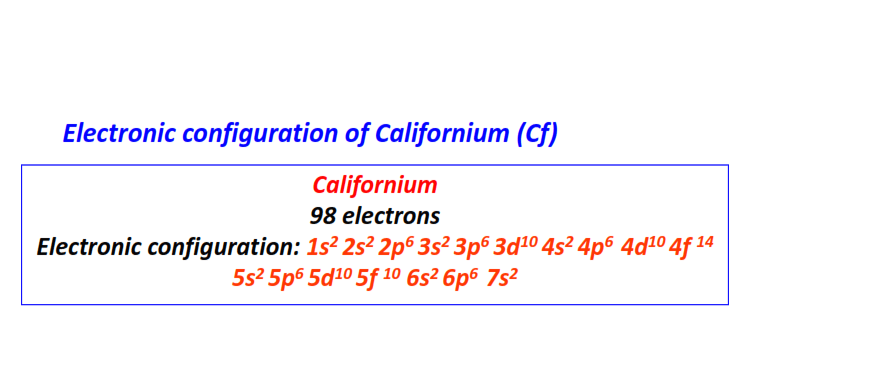
| 98 | Californium (Cf) | 1s22s22p63s23p63d104s24p64d104f145s25p65d105f106s26p67s2
|
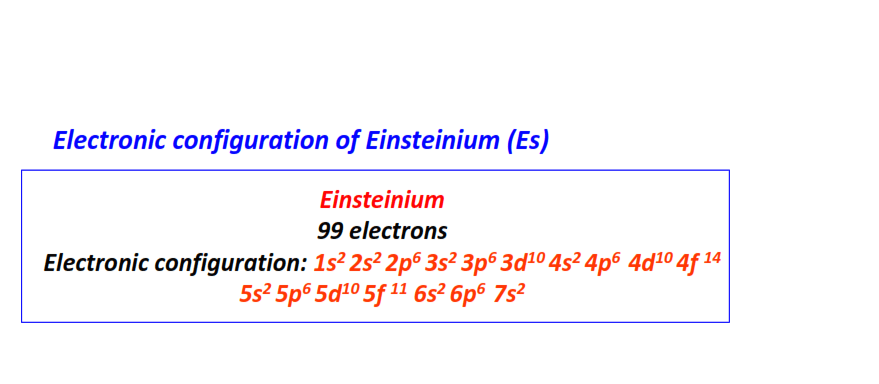
| 99 | Einsteinium (Es) | 1s22s22p63s23p63d104s24p64d104f145s25p65d105f116s26p67s2
|
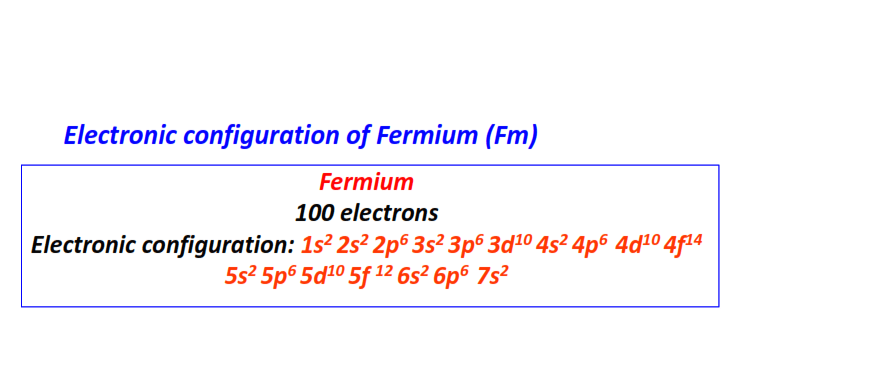
| 100 | Fermium (Fm) | 1s22s22p63s23p63d104s24p64d104f145s25p65d105f126s26p67s2
|
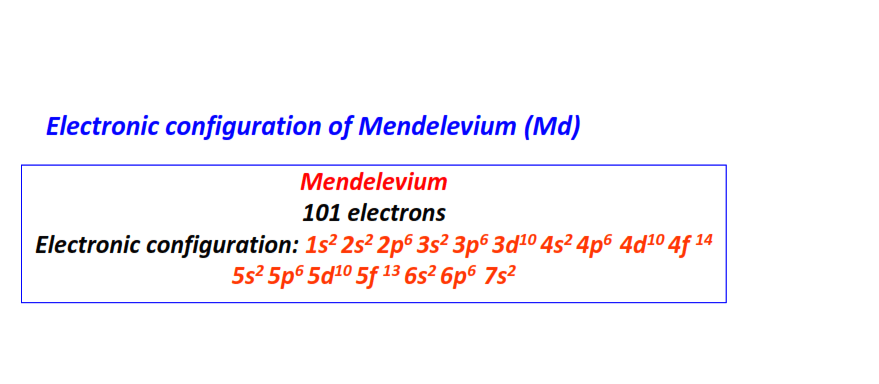
| 101 | Mendelevium (Md) | 1s22s22p63s23p63d104s24p64d104f145s25p65d105f136s26p67s2
|
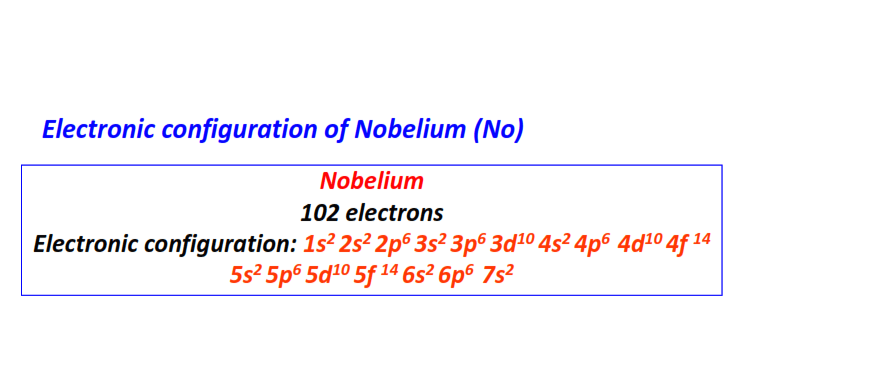
| 102 | Nobelium (No) | 1s22s22p63s23p63d104s24p64d104f145s25p65d105f146s26p67s2
|
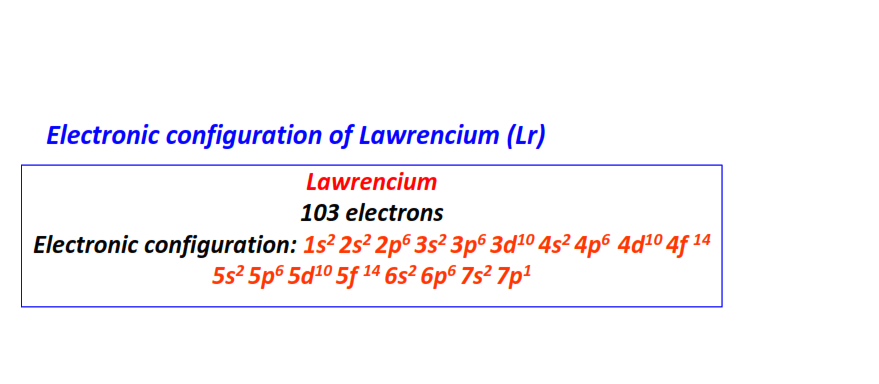
| 103 | Lawrencium (Lr) | 1s22s22p63s23p63d104s24p64d104f145s25p65d105f146s26p67s27p1
|
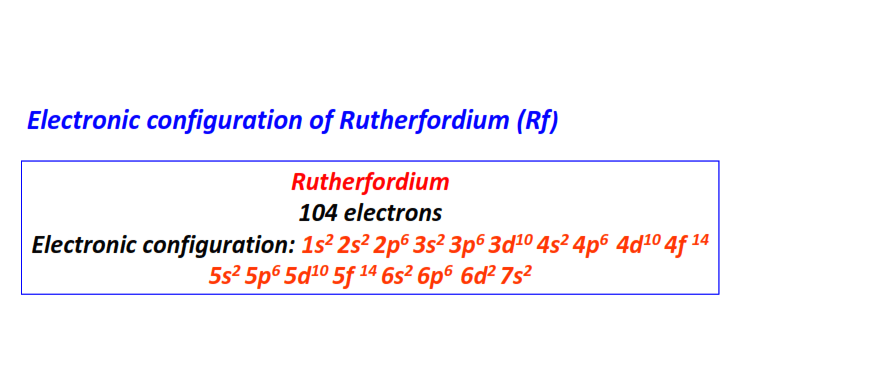
| 104 | Rutherfordium (Rf) | 1s22s22p63s23p63d104s24p64d104f145s25p65d105f146s26p66d27s2
|
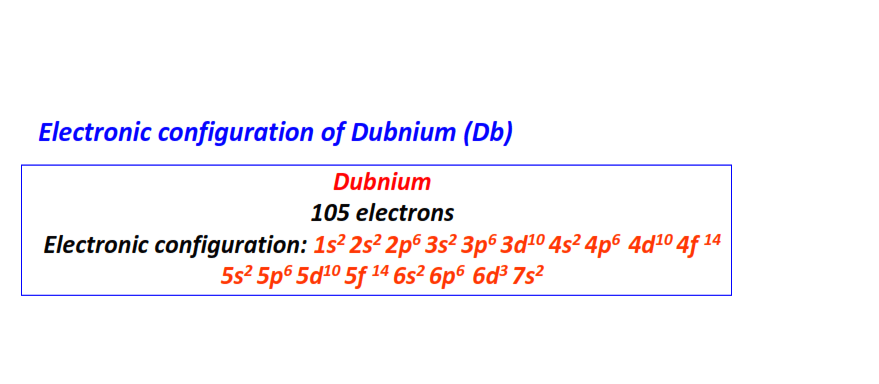
| 105 | Dubnium (Db) | 1s22s22p63s23p63d104s24p64d104f145s25p65d105f146s26p66d37s2
|
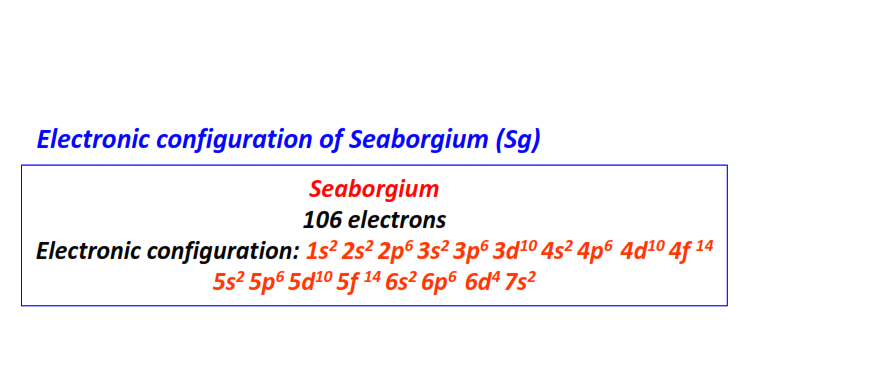
| 106 | Seaborgium (Sg) | 1s22s22p63s23p63d104s24p64d104f145s25p65d105f146s26p66d47s2
|
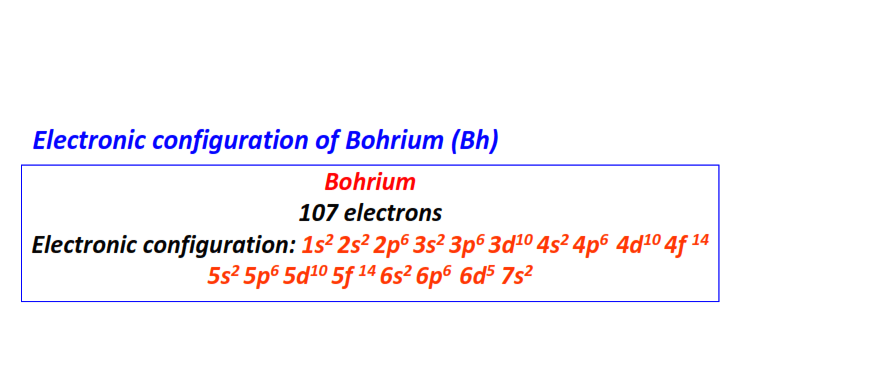
| 107 | Bohrium (Bh) | 1s22s22p63s23p63d104s24p64d104f145s25p65d105f146s26p66d57s2
|
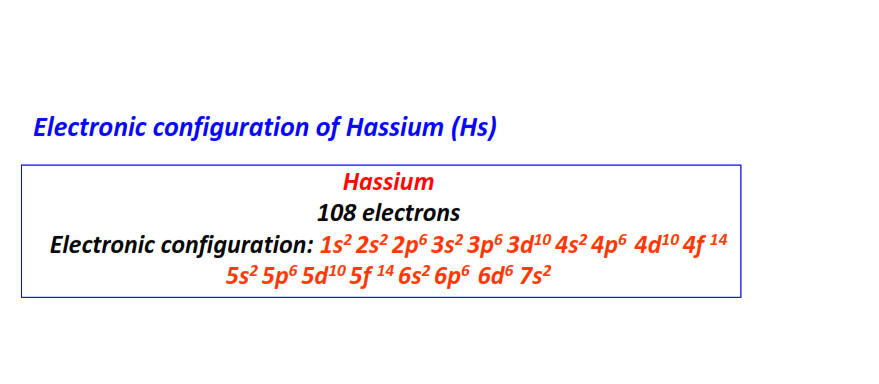
| 108 | Hassium (Hs) | 1s22s22p63s23p63d104s24p64d104f145s25p65d105f146s26p66d67s2
|
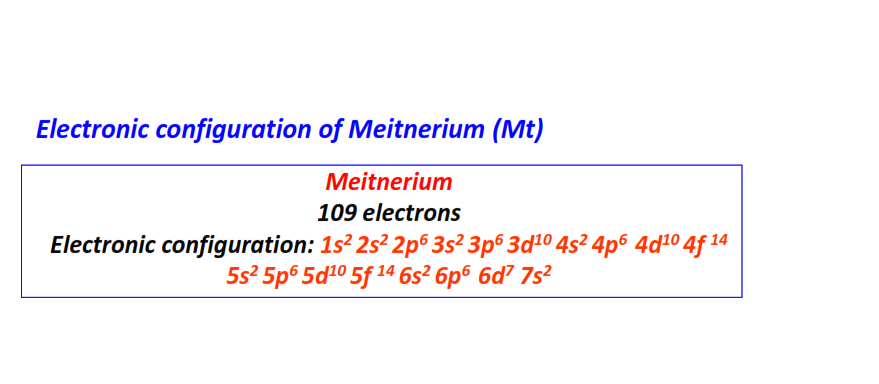
| 109 | Meitnerium (Mt) | 1s22s22p63s23p63d104s24p64d104f145s25p65d105f146s26p66d77s2
|
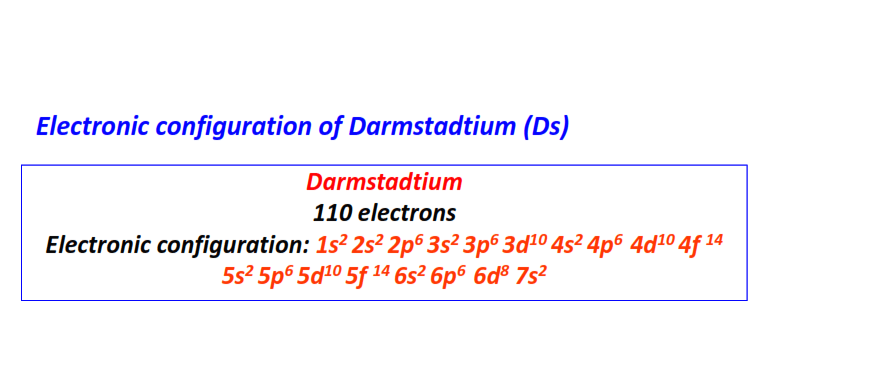
| 110 | Darmstadtium (Ds) | 1s22s22p63s23p63d104s24p64d104f145s25p65d105f146s26p66d87s2
|
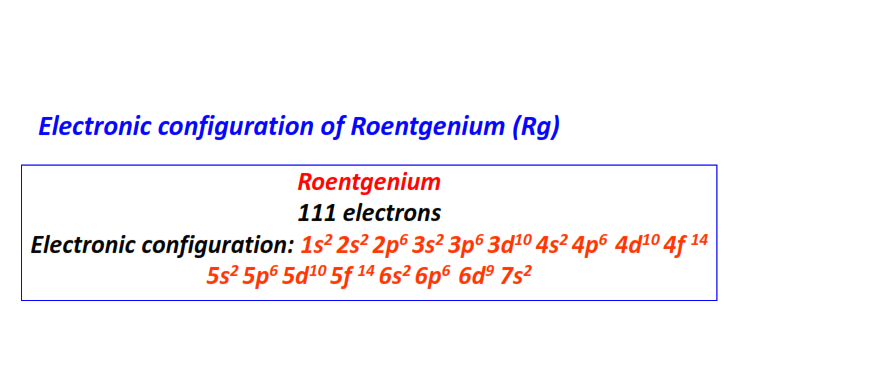
| 111 | Roentgenium (Rg) | 1s22s22p63s23p63d104s24p64d104f145s25p65d105f146s26p66d97s2
|
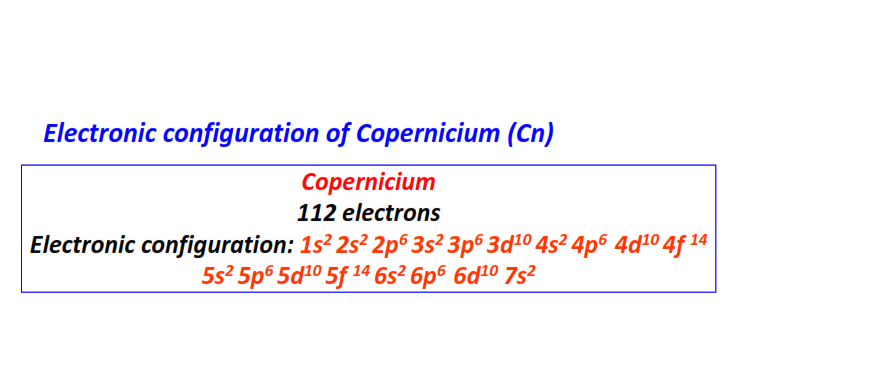
| 112 | Copernicium (Cn) | 1s22s22p63s23p63d104s24p64d104f145s25p65d105f146s26p66d107s2
|
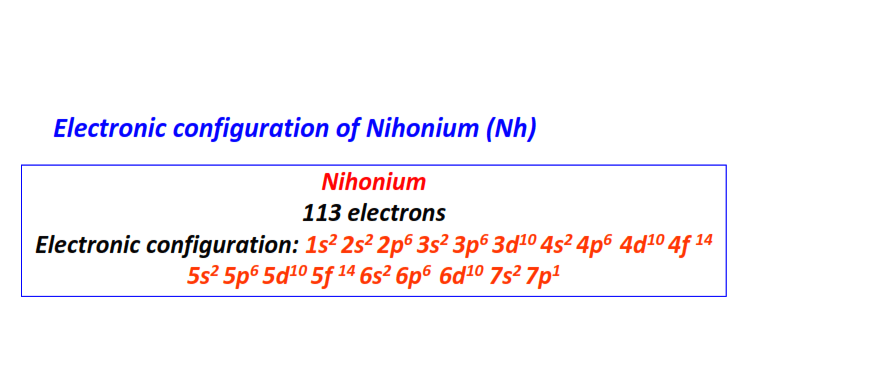
| 113 | Nihonium (Nh) | 1s22s22p63s23p63d104s24p64d104f145s25p65d105f146s26p66d107s27p1
|
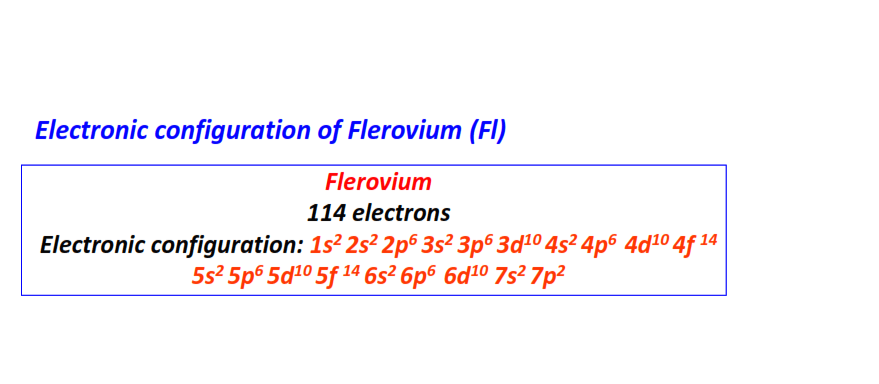
| 114 | Flerovium (Fl) | 1s22s22p63s23p63d104s24p64d104f145s25p65d105f146s26p66d107s27p2
|
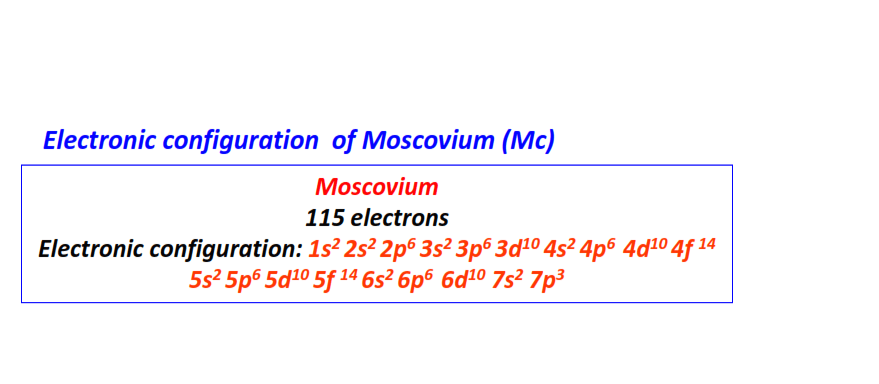
| 115 | Moscovium (Mc) | 1s22s22p63s23p63d104s24p64d104f145s25p65d105f146s26p66d107s27p3
|
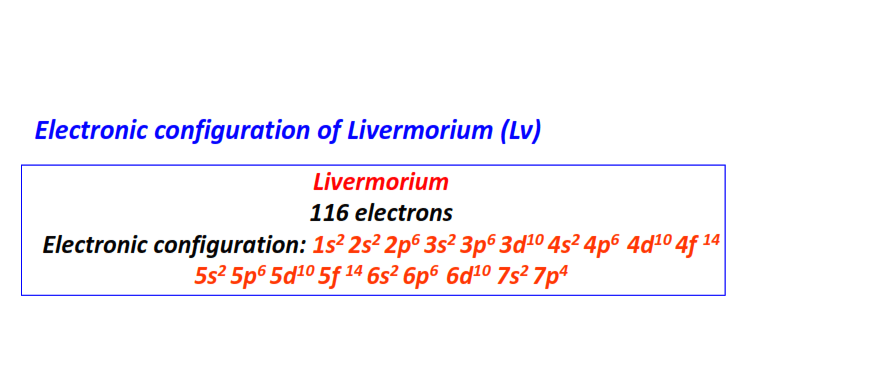
| 116 | Livermorium (Lv) | 1s22s22p63s23p63d104s24p64d104f145s25p65d105f146s26p66d107s27p4
|
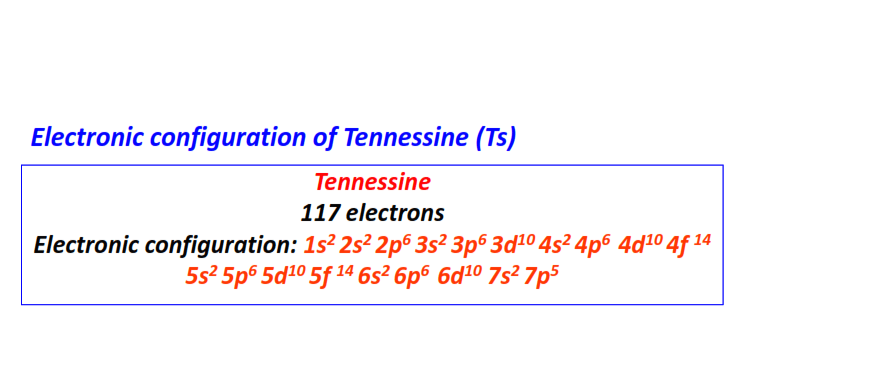
| 117 | Tennessine (Ts) | 1s22s22p63s23p63d104s24p64d104f145s25p65d105f146s26p66d107s27p5
|
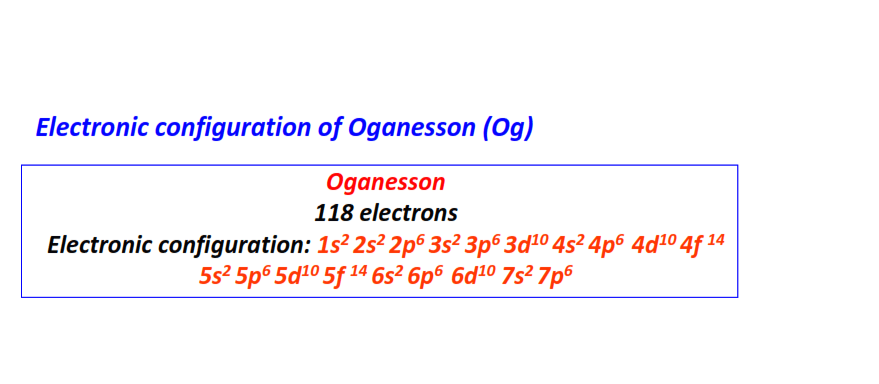
| 118 | Oganesson (Og) | 1s22s22p63s23p63d104s24p64d104f145s25p65d105f146s26p66d107s27p6
|
In conclusion, understanding the electron configurations of elements is crucial for understanding the fundamental structure of matter. This article has provided a list of the electron configurations of all 118 elements in the periodic table, organized by atomic number and presented in a clear tabular format.
We hope that this guide has served as a valuable resource and has helped to enhance your understanding of electron configurations.
Also check:
- How to write the electron configuration for any elements?
- Abbreviated or Noble gas electron configuration calculator
- Electron configuration calculator
- Ion electron configuration calculator
References
- Wikipedia, (n.d.). Electron configurations of the elements (data page). [online] Available at: https://en.wikipedia.org/wiki/Electron_configurations_of_the_elements_(data_page) [Accessed 16-09-2023].
About the author
Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. The team at Topblogtenz includes experts like experienced researchers, professors, and educators, with the goal of making complex subjects like chemistry accessible and understandable for all. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/