So, the question is – Choose the best lewis structure for OCl2?
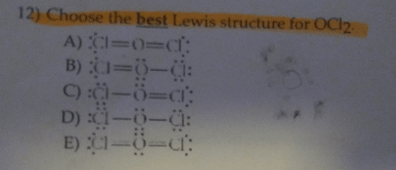
According to the above figure, option – (D) is the best lewis structure of OCl2.
- The best lewis structure of OCl2 has an oxygen (O) atom at the central position, the two chlorine (Cl) atoms are bonded to this central atom with the help of two single bonds.
- In the correct lewis structure of OCl2, 2 lone pairs on the oxygen atom, and 3 on each chlorine atom are present.
|
Explanation –
The lesser the formal charge on atoms, the better the stability of the lewis diagram. We need to check the formal charge on each lewis structure of OCl2 to get its stability.
The formal charge can be found by using the formula given below –

Let’s calculate the formal charge for this lewis structure of OCl2 (Option D) –

For chlorine atom:
⇒ Valence electrons of chlorine = 7
⇒ Nonbonding electrons on chlorine= 6
⇒ Bonding electrons around chlorine(1 single bond) = 2
∴ (7 – 6 – 2/2) = 0 formal charge on chlorine atoms.
For oxygen atom:
⇒ Valence electrons of oxygen = 6
⇒ Nonbonding electrons on oxygen = 4
⇒ Bonding electrons around oxygen(2 single bond) = 4
∴ (6 – 4 – 4/2) = 0 formal charge on oxygen atoms.
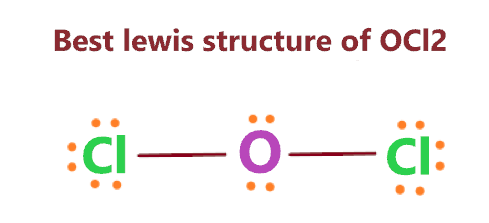
So, all atoms in the above structure get a formal charge equal to zero, hence, this is our most stable and correct lewis structure of OCl2.
Also, you can easily calculate the formal charge for every lewis structure, and then choose the best structure that has the least or zero formal charge.
Also check –




