Scandium (Sc) Electron configuration, Orbital diagram, and Valence electrons
Scandium has an atomic number of 21 and belongs to Group 3 also known as the transition metals group. It is situated in the d-block of the periodic table. Scandium has the symbol Sc and it is classified as a rare earth element.
In this article, we will discuss – Scandium Electron configuration, Orbital diagram, and Valence electrons in detail.
Orbital diagram:- A orbital diagram is simply a pictorial representation of the arrangement of electrons in the orbital of an atom, it shows the electrons in the form of arrows, also, indicates the spin of electrons.
Electron configuration:- Electron configuration is the arrangement of electrons in atomic orbitals. It shows the electrons in numbers, It doesn’t show the details on the spin of electrons like the orbital diagram.
Valence electrons:- Valence electrons are the simply outermost electron of an atom situated in an outermost shell surrounding an atomic nucleus. They can participate in the formation of chemical bonds.
Electron configuration, Valence electrons, and Orbital diagram of Scandium in tabular form
| Name of atom | Scandium (Sc) |
| Number of electrons | 21 |
| Number of electrons per shell | [2, 8, 9, 2] |
| Number of valence electrons | 3 |
| Electron configuration | 1s22s22p63s23p64s23d1 or [Ar] 4s23d1 |
| Orbital diagram | Consists of seven orbitals – 1s, 2s, 2p, 3s, 3p, 4s, and 3d. |
How to find Electron configuration of Scandium (Sc)?
The electron configuration of Scandium can be found using the Aufbau principle.
Aufbau Principle:
- The Aufbau rule simply gives the order of electrons filling in the orbital of an atom in its ground state.
- It states that the orbital with the lowest energy level will be filled first before those with high energy levels. In short, the electrons will be filled in the orbital in order of their increasing energies.
- For example, the 1s orbital will be filled first with electrons before the 2s orbital.
Simply understand that there are commonly four different types of subshells – s, p, d, and, f.
These subshells can hold a maximum number of electrons on the basis of a formula, 2(2l + 1) where ‘l’ is the azimuthal quantum number.
Value of ‘l’ for different subshells.
| Subshells | Value of ‘l’ | Maximum number of electrons, 2(2l + 1) | Number of orbitals in the subshell |
| s | 0 | 2 | 1 |
| p | 1 | 6 | 3 |
| d | 2 | 10 | 5 |
| f | 3 | 14 | 7 |
So, in short, the s subshell can hold a maximum of 2 electrons(1 orbital), the p subshell can hold 6 electrons(3 orbitals), the d subshell can hold 10 electrons(5 orbitals), and the f subshell can hold at most 14 electrons(7 orbitals).
Generally, (n + l) rule is used to predict the energy level of subshells.
n = principle quantum number
l = Azimuthal quantum number
⇒ Lower the value of (n + l) for an subshell, the lower its energy, hence, it will be filled first with electrons.
⇒ For two different subshells having same (n + l) value, then the subshell with lower value of n has lower energy.
So, all these are basics of How filling of electrons will be done in different subshells, obviously, you don’t have so much time for writing electron configuration by using so many rules.
Therefore, we have a diagonal rule for electron filling order in the different subshells using the Aufbau principle.
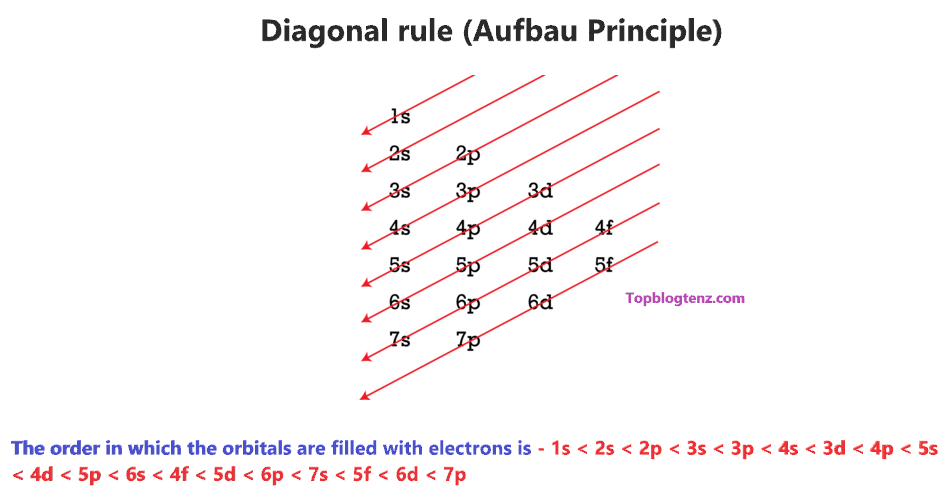
So, the order in which the orbitals are filled with electrons from lower energy to higher energy is – 1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s < 4f < 5d < 6p < 7s < 5f < 6d < 7p and so on.
Now, the electron configuration of an atom can be built by filling the electrons in a lower energy subshell first then higher, higher, and higher.
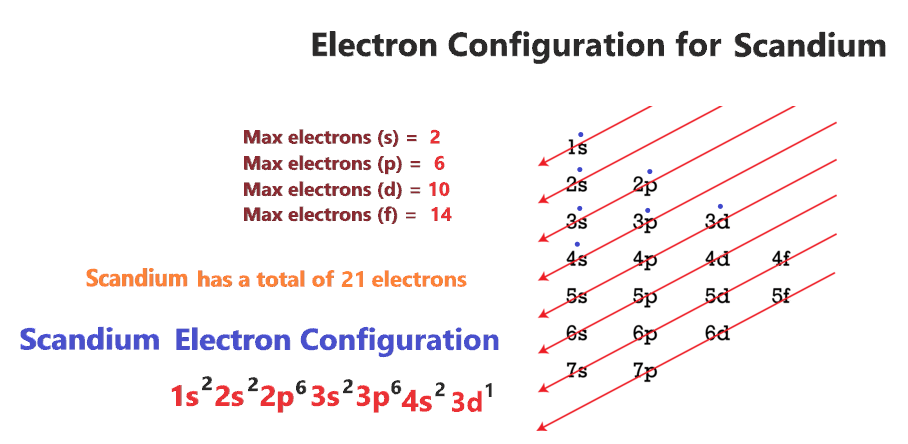
Scandium Electron configuration using the Aufbau Principle
- A Scandium atom is a neutral atom that has an atomic number of 21 which implies it has a total of 21 electrons.
- As per the Aufbau rule, the electrons will be filled into 1s orbital first then 2s, then 2p…so on.
- Now, for the electron configuration of Scandium, the first 2 electrons will go in 1s orbital since s subshell can hold a maximum of 2 electrons.
- The next two electrons will go into the 2s orbital, after that, the next 6 electrons will go into the 2p orbital since the p subshell can hold up to 6 electrons.
- The next two electrons will go into the 3s orbital, after that, the next six electrons will go into the 3p orbital and the next two electrons in the 4s orbital, finally, the remaining one electron will go into the 3d orbital.
- Therefore, the electron configuration of Scandium will be 1s22s22p63s23p64s23d1.
It should be noted that –
As per (n + l) rule, 4s has a value n = 4 and l = 0, therefore, n + l = 4
On the other hand, the 3d has a value n = 3 and l = 2, therefore, n + l = 5
So, according to Aufbau principle – the 4s has lower energy than 3d orbital, therefore, 4s orbital should be filled first than 3d orbital.
Therefore, the correct electron configuration for scandium according to energy level is – 1s22s22p63s23p64s23d1
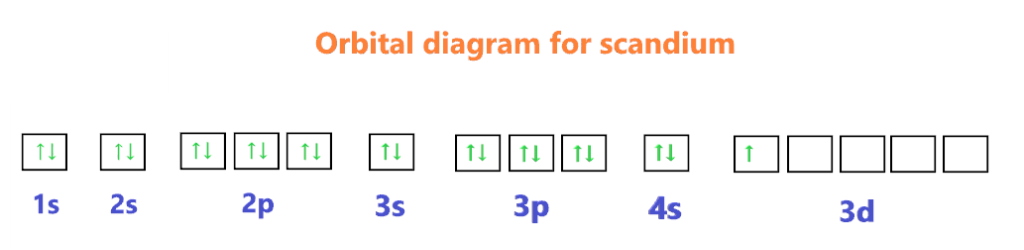
Orbital diagram for Scandium
The orbital diagram simply represents the arrangement of electrons in the different orbitals of an atom, it uses an arrow to represent the electrons, every orbital(one box) contains a maximum of 2 electrons.
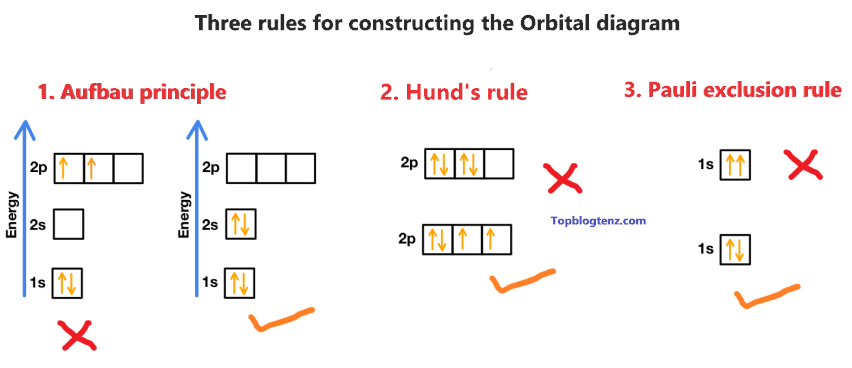
There are three rules followed for constructing the orbital diagram for an atom.
(1). Aufbau’s principle:- This rule state that the lower energy orbital will be filled before the higher energy orbital, for example – the 1s orbital will fill before the 2s orbital.
(2). Hund’s rule:- This rule state that each orbital of a given subshell should be filled with one electron each before pairing them. That means “Each orbital gets one electron first, before adding the second electron to the orbital”.
(3). Pauli Exclusion Principle:- This rule state that, no two electrons can occupy the same orbital with the same spin. That means “One must be spin up (↑) and one must be spin down (↓)”.
If you understand the above rules then constructing the orbital diagram or orbital notation for Scandium is super easy.
Basics of Orbital diagram:-
There are different types of orbitals – s, p, d, and, f. These orbitals contain a number of boxes that can hold a number of electrons. Let’s see.
Each box will hold a maximum of 2 electrons with opposite spin.
- S orbital contains 1 box that can hold a maximum of 2 electrons.
- P orbital contains 3 boxes that can hold a maximum of 6 electrons.
- D orbital contains 5 boxes that can hold a maximum of 10 electrons.
- F orbital contains 7 boxes that can hold a maximum of 14 electrons.
The orbital diagram will also be filled with the same order as described by the Aufbau principle. (1s < 2s < 2p < 3s……and so on.)
Also check –
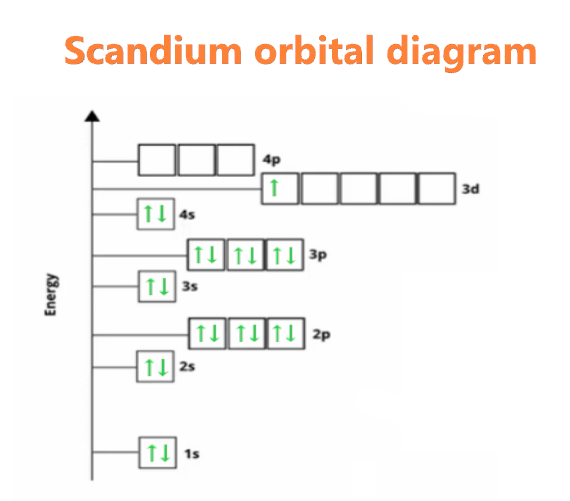
What is the Orbital diagram for Scandium?
We know the electron configuration of Scandium is 1s22s22p63s23p64s23d1, now for drawing its orbital diagram, we need to show its electrons in form of an arrow in different boxes using Hund’s and Pauli’s exclusion rule.
- The orbital diagram of Scandium contains 1s orbital, 2s orbital, 2p orbital, 3s orbital, 3p orbital, 4s orbital, and 3d orbital.
- 1s orbital contains 1 box, 2s orbital also contains 1 box, 2p orbital contains 3 boxes, 3s orbital contains 1 box, 3p orbital contains 3 boxes, 4s orbital contains 1 box and 3d orbital contains 5 boxes.
- Scandium has a total of 21 electrons and one box can hold up to two electrons.
- Therefore, the first two electrons will go into the 1s orbital, the next two will go into the 2s orbital, and after that, the next six electrons will go into the 2p orbital, since, the 2p orbital has 3 boxes.
- After that, the next two electrons will go into the 3s orbital, and the next six electrons will enter the 3p orbital. Now, the 3p orbital is full.
- Therefore, the two electrons will go in the 4s orbital and the remaining one electron in the 3d orbital box.
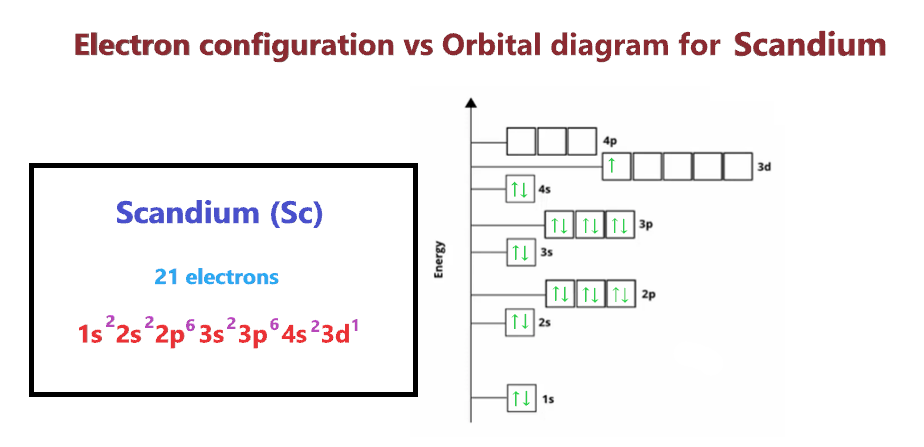
Electron configuration Vs Orbital diagram for Scandium
The main difference between the orbital diagram and electron configuration is an orbital diagram shows electrons in form of arrows whereas an electron configuration shows electrons in form of numbers. Also, the orbital diagram shows details on the spin of electrons whereas the electron configuration doesn’t show it.
Both these follow the Aufbau principle (Diagonal rule).
Also Read:–
Scandium Valence electrons
Valence electrons are the outermost electrons present in the outermost shell of an atom. They have more energy, hence, they are part of most chemical reactions.
We can find the valence electrons of an atom by using its electron configuration.
How to find valence electrons of Scandium atom
We know, the electron configuration of the Scandium atom is 1s22s22p63s23p64s23d1, and valence electrons are those electrons found in the outer shell of an atom.
Note:
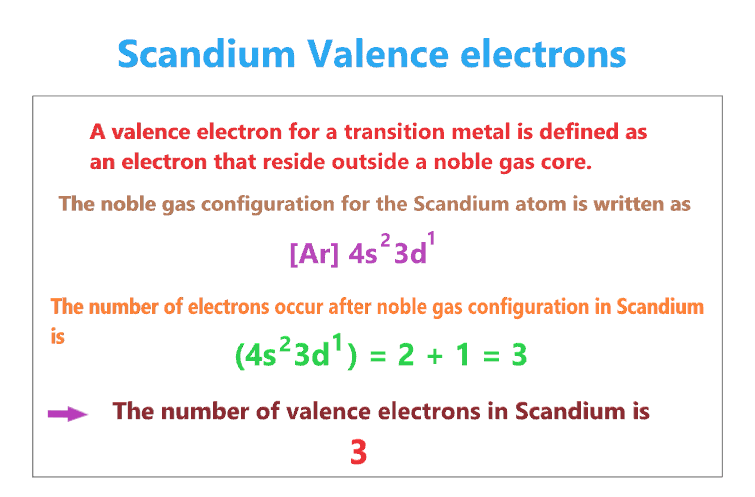
A valence electron for a transition metal is defined as an electron that reside outside a noble gas core.
Or
For transition metals, the number of valence electrons is equal to the number of electrons that occur after a noble-gas configuration.
Look at the article – How to find valence electrons of transition metals (d block elements)?
So, by this concept, we can easily find the valence electrons for the scandium atom.
- The noble gas configuration for the Scandium atom is written as [Ar] 4s23d1.
- So, the number of electrons occur after noble gas configuration in Scandium is (4s23d1) = 2 + 1 = 3
- Therefore, the number of valence electrons in Scandium is 3.
Check – Valence electron calculator to calculate the number of valence electrons for any atom
Also read:
- Nitrogen orbital diagram and electron configuration
- Oxygen orbital diagram and electron configuration
- Carbon orbital diagram and electron configuration
- Fluorine orbital diagram and electron configuration
- Neon orbital diagram and electron configuration
- Boron orbital diagram and electron configuration
- Sodium orbital diagram and electron configuration
- Magnesium orbital diagram and electron configuration
- Aluminum orbital diagram and electron configuration
- Silicon orbital diagram and electron configuration
- Phosphorous orbital diagram and electron configuration
- Sulfur orbital diagram and electron configuration
- Chlorine orbital diagram and electron configuration
- Argon orbital diagram and electron configuration
- Potassium orbital diagram and electron configuration
- Beryllium orbital diagram and electron configuration
- Lithium orbital diagram and electron configuration
FAQ
How do you write scandium electron configuration?
|
What is the shorthand electron configuration of Scandium?The shorthand electron configuration for the Scandium atom is [Ar] 4s23d1. ∴ [Ar] electron configuration is 1s22s22p63s23p6. |
Which element has the 1s22s22p63s23p64s23d1 Electron configuration?Element with electron configuration 1s22s22p63s23p64s23d1 is Scandium (Sc) that has the atomic number of 21. |
How many valence electrons does Scandium have?The Scandium atom has total of 3 valence electrons. |
What is the orbital diagram for Scandium (Sc)?The orbital diagram for Scandium is drawn with 7 orbitals. The orbitals are 1s, 2s, 2p, 3s, 3p, 4s, and 3d. The Scandium orbital diagram contains 2 electrons in the 1s orbital, 2 electrons in the 2s orbital, the six electrons in the 2p orbital, the two electrons in the 3s orbital, and the next six electrons in the 3p orbital, the next two electrons in 4s orbital, and the remaining one electron in 3d orbital. The orbital diagram for a ground-state electron configuration of a Scandium atom is shown below-
|
What is the electron configuration of the Sc3+ ion?We know, in general, the electron configuration of Scandium (Sc) is 1s22s22p63s23p64s23d1. Now, in the Sc3+ ion, the positive charge means, that Scandium loses three electrons. Therefore, to write the electron configuration of the SC3+ ion, we have to remove three electrons from the configuration of Scandium (Sc). ∴ The resulting electron configuration for the Scandium ion (Sc3+) will be 1s22s22p63s23p6. It resembles the configuration of the nearest inert gas i.e Argon. |
Summary
- The electron configuration of Scandium in terms of the shell or orbit is [2, 8, 9, 2].
- The ground-state electron configuration of the Scandium (Sc) atom is 1s22s22p63s23p64s23d1.
- The shorthand electron configuration for Scandium is [Ar] 4s23d1.
- The electron configuration for Scandium ion (Sc3+) is 1s22s22p63s23p6.
- The number of valence electrons available for the Scandium atom is 3. Scandium is situated in the transition metal group and has an atomic number of 21.
- The orbital diagram for Scandium is drawn by following three principles – the Aufbau principle, Hund’s principle, and Pauli’s exclusion principle.
- The Scandium orbital diagram comprises seven orbitals. The seven orbitals are 1s, 2s, 2p, 3s, 3p, 4s, and 3d.
- The first two electrons will go in the 1s orbital, the next two in the 2s orbital, the next six in the 2p orbital, the next two electrons in the 3s orbital, the next six electrons in the 3p orbital, the next two in the 4s orbital and the remaining one electron in 3d orbital.
About the author
Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. The team at Topblogtenz includes experts like experienced researchers, professors, and educators, with the goal of making complex subjects like chemistry accessible and understandable for all. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/