Is Ammonium hydroxide (NH4OH) an acid or base? - Weak or Strong

Ammonium hydroxide is a solution of ammonia in water that has the chemical formula NH4OH. It appears colorless and able to irritate the eyes on direct contact. It is also known as ammonia water, ammonia liquor, and aqueous ammonia.
In this article, we will discuss Is Ammonium hydroxide (NH4OH) an acid or base? Is it strong or weak, etc?
So, Is NH4OH an acid or base? NH4OH is a base. Because it is simply an aqueous solution of ammonia that contains two ions (NH4+ and OH–) and anything that has hydroxide ions in an aqueous solution is considered as the base in nature. The pH value of NH4OH lies between 7 to 10.
| Name of Molecule | Ammonium hydroxide |
| Chemical formula | NH4OH |
| Molar mass | 35.04 g·mol−1 |
| Nature | Weak base |
| pH value | 7-10 |
Why NH4OH is an base?
To Know Why NH4OH is acting as a Base? We have to look into the famous theory given by Arrhenius for the base compounds.
According to Arrhenius theory, the compound is said to be Arrhenius base when it produces OH– ion through ionization or through dissociation in water and increases the concentration of OH– ions in an aqueous solution.
Now have a look at NH4OH dissociation in an aqueous solution.
⇒ NH4OH(aq) ⇔ NH4+(aq) + OH−(aq)
When ammonium hydroxide is dissolved in water, the ion-water attraction overcomes the attraction between ions, so it dissociates into the ammonium cation(NH4+) and hydroxide anion(OH–).
Also, Arrhenius states that a base is a compound that increases the concentration of hydroxide ion (OH–) in solution.
Ammonium hydroxide is itself a solution of ammonia in water that contains OH– ions, hence, we can say, NH4OH is an Arrhenius base.
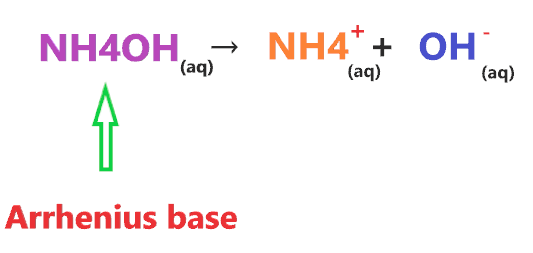
Now we look for another most important acid-base theory which is the Bronsted-Lowry theory.
This theory states a compound is classified as a base when it accepts the proton from another compound.
- A Bronsted-Lowry acid is a proton (hydrogen ion) donor.
- A Bronsted-Lowry base is a proton (hydrogen ion) acceptor.
Let’s check whether NH4OH fulfills the requirement for classifying as Bronsted-Lowry base or not.
Consider the reaction of NH4OH with HCl.
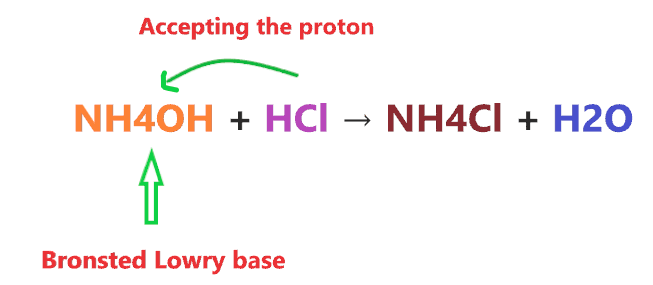
Clearly, when NH4OH reacts with a strong acid like HCl, it accepts the one proton from it, hence, according to the above definition, NH4OH will act as a Bronsted-Lowry base.
Is Ammonium hydroxide (NH4OH) strong base or weak base?
To know whether Ammonium hydroxide (NH4OH) is a strong base or weak, you must know the basic difference between a strong base and a weak base.
Strong base: A compound is a strong base when it completely dissociates in an aqueous solution and liberates a large number of hydroxide ions. All moles of the strong base dissociate into hydroxide ion (OH-) and no part remains undissociated in the solution.
Example: Sodium hydroxide(NaOH), Barium hydroxide (Ba(OH)2), Calcium hydroxide (Ca(OH)2), Lithium hydroxide (LiOH), Potassium hydroxide (KOH), etc.
Also, Read:–
- Why KOH is a strong base?
- Why LiOH is a strong base?
- Why Ba(OH)2 is a strong base?
- Why Ca(OH)2 is a strong base?
- Why NaOH is a strong base?
Weak base: A compound is a weak base when it partially or not completely dissociates in an aqueous solution. It means only some of the weak base dissociates in the solution to produce OH– ion, and at equilibrium, both undissociated base and their ionized product are present in the solution.
Example- Ammonia (NH3), Methylamine (CH3NH2), etc.
Also, Read:–
So, Is Ammonium hydroxide (NH4OH) a strong base or a weak base? NH4OH is considered a weak base. Because it is an aqueous solution of ammonia which is also a weak base that means ” only a tiny fraction of ammonia reacts with water to form ammonium ion and hydroxide ion. The vast majority just stays as NH₃“.

Note: NH4OH is a combination of NH3 + H2O, hence, not to be confused.
As shown in the figure, when NH3 is dissolved in water (NH3 + H2O = NH4OH), it dissociates into two ions (NH4+ and OH–) but the ion (NH4+) is not stable in an alkaline environment, it keeps breaking into NH3 and H+.
⇒ NH4+ ⇔ NH3 + H+
The H+ ions react with OH– and forms H2O.
⇒ NH3 + H2O ⇔ NH4+ + OH–
The double arrow in the reaction of ammonia and water shows that both forward and backward reactions occur at equilibrium.
But the the favor of reaction mostly lies to the left side that means large number of NH3 will present in the aqueous solution as compare to its right sided product(NH4+ and OH–)
Hence, the ions (NH4+ and OH–) are very less in number as compared to (NH3 and H2O), therefore, less number of OH– ions makes the ammonium hydroxide a weak base (NH4OH) in nature or you can say, an aqueous solution of ammonia is a weak base due to fewer OH– ions.
Another reason is that the relative size of NH4+ and hydroxide ions are comparable, hence, this leads strong bond between them and preventing from complete dissociation in water.
Hence, NH4OH finds difficulty in full dissociation, however, it partially dissociates but the amount of OH– ions are produced in the final solution is low, hence, NH4OH considered a weak base or weak alkali.
Let’s understand why NH4OH acts as the weak base with the help of the dissociation constant value concept.
⇒ If the value of the dissociation constant of acid is greater than 1 (Ka > 1), then the nature of the compound is a strong acid.
⇒ If Ka < 1, then the nature of the compound is a weak acid.
⇒ If Kb >1, then the nature of the compound is a strong base.
⇒ If Kb <1, then the nature of the compound is a weak base.
∴ The base dissociation constant value(Kb) for NH4OH is 1.8 × 10-5 that’s way lower than recommended value for the Strong base, hence, NH4OH is a weak base in nature.
It should be noted that NH4OH is not a real compound, it’s just a solution of ammonia in water.
Ammonia is a weak base, according to the hydrolysis reaction:
NH3 + H2O ⇔ NH4+ + OH–, Kb = 1.8 × 10-5
Please notice that Kb is quite small, meaning that very little ammonium and hydroxide forms in the solution. In other words, the concentration of OH– is much smaller than the nominal ammonia or “ammonium hydroxide” concentration. That’s why … weak base.
Not because NH4OH molecules fails to dissociate — there are no NH4OH molecule. [source]
Here’s the list of some common acids and bases with their strength.

Also check:-
Uses of Ammonium hydroxide
- Ammonium hydroxide has a role as a food acidity regulator.
- It is used as a cleansing agent in many household works.
- It is also used as a sanitizer in industrial cleaners.
- It is used in the manufacturing process of chemical fertilizers.
- It is used in the production of organic and inorganic chemicals.
- It is also used in furniture darkening.
- It is used for various manufacturing products such as Plastic, Rubber, fertilizer. and rayon, etc.
Properties of Ammonium hydroxide
- It is a colorless liquid solution of ammonia.
- It has a highly pungent odor.
- It has a boiling point of 37.7 °C and a melting point of −57.5 °C.
- It is miscible in water.
- Its standard enthalpy of formation is -80 Kilojoules per mole.
- It can cause severe irritation on direct contact with the skin for the long term.
Summary
Ammonium hydroxide is a solution of ammonia in water that appears as a colorless liquid with a highly pungent odor having a pH value between 7 to 10. It has a molar mass of 35.04 g/mol. It is often denoted by the symbol NH3 (aq) and is also known as ammonia water or aqueous ammonia. At last, with some important points of this article on Is NH4OH an acid or base? we will finish it.
- Is Ammonium hydroxide (NH4OH) an acid or base? NH4OH is a base. It is an aqueous solution of ammonia that contains two ions (NH4+ and OH–). The hydroxide ions in an aqueous solution show the basic nature. Therefore, Ammonium hydroxide (NH4OH) is a base, with a pH lies between 7 to 10.
- NH4OH acting as Arrhenius base and Bronsted-Lowry base.
- The base dissociation constant value (Kb) for NH4OH is 1.8 × 10-5 that’s way lower than recommended value for the Strong base, hence, NH4OH is a weak base in nature.
- Ammonium hydroxide (NH4OH) is a weak base. Because the production of the number of OH– ions are very low as compared to the number of NH3 we dissolved in water, this is because ammonia is itself a weak base, and it’s only a tiny fraction of it reacts with water to form(NH4+ and OH–), hence, an only small fraction of OH– ions are produced in solution.
About the author
Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. The team at Topblogtenz includes experts like experienced researchers, professors, and educators, with the goal of making complex subjects like chemistry accessible and understandable for all. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/