Is Sodium cyanide (NaCN) an acid or base or neutral?

Sodium cyanide is a poisonous compound that appears as a white crystalline solid and smells like faint almonds. It has the chemical formula NaCN. It has the capability of inhibiting metabolic processes due to its extremely poisonous nature.
In this article, we will discuss Is Sodium cyanide (NaCN) is an acid or base or salt?
So, Is NaCN an acid or base? NaCN is neither an acid nor base. It is formed from the neutralization of a strong base, namely Sodium hydroxide (NaOH), and a weak acid, namely Hydrocyanic acid (HCN), however, NaCN is said to be basic salt, due to the formation of OH– radicals when dissolved in aqueous solution.
| Name of Molecule | Sodium cyanide |
| Chemical formula | NaCN |
| Molar mass | 49.007 g/mol |
| Nature | Basic salt |
| pH value | >7 |
Why NaCN can't be acid in nature?
A compound is said to be acid when it is capable of releasing the proton or H+ ion on dissolving in an aqueous solution. According to Arrhenius’s theory of acid ” A compound is said to be acid when added in water, it increases the number of H+ ions in solution.
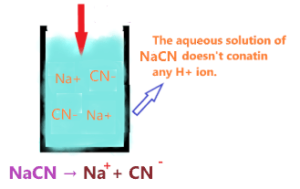
Since NaCN doesn’t contain any hydrogen ion, it is unable to release the proton in an aqueous solution.
So, as per Arrhenius’s theory, an aqueous solution of NaCN doesn’t have any property of acid due to the absence of an H+ ion. Therefore, we can say NaCN is not an Arrhenius acid.
Bronsted-Lowry’s theory for an acid state that ” A compound is said to be acid that donates the proton to other species and make conjugate base”.
Therefore, once again NaCN doesn’t have any proton, so obvious it is not able to donate the proton to other species.
Hence, NaCN is also not a Bronsted-Lowry acid.
Why NaCN is a basic salt?
Have you ever heard what these terms mean by “Acidic salt”, “Basic salt”, “Neutral salt”, or “Amphoteric salt”?
Acidic salt: Acidic salt is formed when a neutralization reaction carries out between strong acid and weak base. This type of salt produces an acidic solution after being dissolved in a solvent that has a pH value of less than 7. Examples of acidic salt – NH4Cl, NH4NO3, NH4Br, etc.
Also Read:-
Basic salt: Basic salt is formed when a neutralization reaction carries out between a strong base and weak acid. This type of salt produces a basic solution after being dissolved in a solvent that has a pH value of more than 7. Examples of basic salt – are Na2CO3, Soap, NaF, etc.
Also Read:-
Neutral salt: This salt has a pH value equal to 7 which means it neither has acidic properties nor basic properties. It is formed when a neutralization reaction carries out between a strong acid and strong base or weak acid and weak base. Examples of neutral salt – NaCl, KCl, NaNO3, etc.
Also Read:-
Amphoteric salt: This salt can act as acidic salt as well as a basic salt. Or “Amphoteric means the compound has the ability to act as an acid with a base and base with an acid”. Examples of Amphoteric salts – NaHCO3, HCO3–, CH3OH, etc.
Also Read:-
- Why does NaHCO3 act as acid as well as the base?
- Why does HCO3– act as acid as well as the base?
- Why does CH3OH act as acid as well as a base?
Ok, Now come to the point Why does NaCN act as basic salt? As we know, Sodium cyanide is formed when NaOH(Strong base) reacts with HCN(Weak acid). They react with each other in the molar ratio of 1:1.
⇒ NaOH + HCN → NaCN + H2O
As per the concepts of salts, a combination of a strong base and weak acid leads to the formation of basic salt.

The nature of salt(acidic or basic) is dependent on the strength of the component in the acid-base reaction.
- An aqueous solution of stronger acid and weak base will acquire more property of acidic rather than a base.
- An aqueous solution of a stronger base and weak acid will acquire more property of basic rather than an acid.
In the case of NaCN, a stronger base (NaOH) reacts with a weaker acid(HCN), so the aqueous solution of its acquires more property of base rather than an acid.
Hence, we can say NaCN is a basic salt in nature.
Why the aqueous solution of NaCN becomes basic?
An aqueous solution having a pH value of more than 7 is called a basic aqueous solution.
When sodium cyanide is dissolved in an aqueous solution, it will split into two ions Na+ and CN–.
⇒ NaCN → Na+ + CN–
The Na+ is a cation of strong base(NaOH), hence, it will not undergo hydrolysis in water and doesn’t affect the pH of the solution. But the CN– is the anion of a weak acid that gets hydrolysis in water to form HCN, leaving OH– ions that make the solution basic.
⇒ CN– + H2O → HCN + OH–

A weak acid like HCN remains undissociated in an aqueous solution or only partially dissociate, hence, the final solution contains more OH– ion than H+ ion.
Therefore, with the presence of additional OH– ions in the aqueous solution, sodium cyanide (NaCN) solution becomes basic in nature.
Note: An aqueous solution nature depends on the H+ and OH– ions. When more OH– ions are present in an aqueous solution, then the solution becomes basic, and when more H+ ions are present in an aqueous solution, the solution becomes acidic.
Also, when the number of H+ and OH– ions same in an aqueous solution, then the solution is said to be neutral.
Also read:–
Is NaCN considered strong or weak(acidic or basic salt)?
Sodium cyanide is neither acidic nor basic, it is salt made up of a strong base and weak acid. Strong bases dissociate completely in solution and release a larges number of OH– ions but the weak acid mostly remain undissociated and release fewer H+ ions.
Therefore, with the presence of more OH– ions in an aqueous solution of NaCN, we can consider it strong basic salt.
The concept of acidic salt and basic salt:-
⇒ Strong base + Weak acid = Basic salt (∴ OH– ions > H+ ions)
⇒ Stronger acid + Weak base = Acidic salt (∴ H+ ions > OH– ions)
Also Check:
Uses of Sodium cyanide
- It is used as a test reagent for the proper functioning of chemoreceptors.
- It is used in gold mining – to extract gold and precious metals in the mining industry.
- It is used in the manufacture of dyes.
- It is used as a chelating agent.
- It is used to produce hydrocyanic acid.
- It is also used to prepare agricultural chemicals.
- It is used in the pharmaceuticals industry.
- It is also used to prepare nitriles.
Properties of Sodium cyanide
- It has a boiling point of 1,496 °C and a melting point of 563.7 °C.
- It has a faint almond-like odor.
- It appears as a white crystalline solid.
- It is soluble in ammonia, methanol, ethanol, etc.
- It is an extremely poisonous compound.
- Sodium cyanide when treated with an acid (such as sulfuric acid), it forms a highly toxic gas known as hydrogen cyanide.
- Its crystal contains a equal number of sodium cation and cyanide anion.
Summary
Sodium cyanide is an extremely poisonous compound also known as sodium salt as well as a one-carbon compound. It is white in color with a faint almond-like odor and highly soluble in water. It is prepared by treating sodium hydroxide with hydrogen cyanide.
- Is sodium cyanide (NaCN) acidic or basic salt? NaCN is a basic salt having a pH value of more than 7, made from the neutralization of a strong base(NaOH) and weak acid (HCN).
- The aqueous solution of sodium cyanide(NaCN) is basic in nature due to having more hydroxide ions produced from the hydrolysis of cyanide ions (CN– + H2O → HCN + OH–).
- The presence of fewer hydrogen ions and more OH– ions, increases the pH level of the NaCN solution.
About the author
Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. The team at Topblogtenz includes experts like experienced researchers, professors, and educators, with the goal of making complex subjects like chemistry accessible and understandable for all. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/

