Is Sodium chloride (NaCl) an acid or base or salt?

Sodium chloride is one of the compounds mostly used in our daily lives. Almost anything we eat has some proportion of this compound. It is commonly known as a salt having the chemical formula NaCl.
Sodium chloride is an ionic compound that appears as a white crystalline solid responsible for the salinity of seawater. It is one of the essential nutrients for animals, plants, and humans also.
It is most commonly used as a food preservative. In this article, we will discuss Is Sodium chloride (NaCl) is an acid or base or neutral?
Is NaCl an acid or base?
NaCl is a neutral salt. It is made from the neutralization reaction of the strong acid, namely Hydrochloric acid (HCl) with a strong base, namely Sodium hydroxide (NaOH). The strong acid and a strong base will neutralize each other effects and a neutral solution forms. Hence, the pH value of the aqueous solution of NaCl is 7.
Let’s dive deep into the topic of Why NaCl can’t be acid or base in nature?
| Name of Molecule | Sodium chloride |
| Chemical formula | NaCl |
| Acid or Base? | Neither acid nor base |
| Molar mass | 58.44 g/mol |
| pH value | 7 |
Why NaCl can't be acid in nature?
A compound is said to be acid when it is capable of releasing the proton or H+ ion on dissolving in an aqueous solution. According to Arrhenius’s theory of acid ” A compound is said to be acid when added in water, it increases the number of H+ ions in solution.
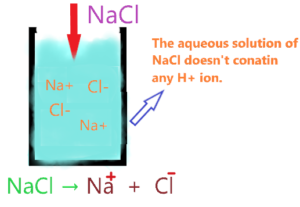
Since NaCl doesn’t contain any hydrogen ion, it is unable to release or donate the proton in an aqueous solution.
So, as per Arrhenius’s theory, NaCl aqueous solution doesn’t have any property of acid due to the absence of H+ ion. Therefore, we can say NaCl is not an Arrhenius acid.
Bronsted-Lowry’s theory for an acid state that ” A compound is said to be acid when dissolved in water they donate the proton to other species and make conjugate base”.
Therefore, once again NaCl doesn’t have any proton, so obvious it is not able to donate the proton to other species.
Hence, NaCl is also not a Bronsted-Lowry acid.
Why NaCl can't be base in nature?
I am sure you are aware of the term “Base” we frequently use in chemistry. “A base is any substance that increases the concentration of OH– ion in aqueous solution”.
NaCl is not a base because it doesn’t have any OH– ion to liberate when dissolved in an aqueous solution.
According to the Bronsted-Lowry theory for base ” A compound is said to be base when it accepts the proton from other species and forms a conjugate acid”.
One might argue NaCl is a Bronsted-Lowry base because one of its ions (Cl–) accepts the proton when dissolved in water and forms a conjugate acid(HCl).
![]()
However, this is not the case, Na+ and Cl– is very very weak acid and base, you can simply treat them as neutral as they don’t have any observable effect on the pH of an aqueous solution.
Cl– is a very weak conjugate base of the strong acid(HCl), hence, it will not react with the either of the H2O ions.
In short, Na+ and Cl– ions are just spectator ions in the solution since they doesn’t take part in chemical reaction.
Therefore, we can say, NaCl is also not a Bronsted-Lowry base as neither of its ions is capable of accepting the proton.
Why NaCl is neutral salt?
“A salt is an ionic compound that contains a cation (base) and an anion (acid).”
According to the concept of salt, when a neutralization reaction carries out between a strong base and weak acid then the salt is formed which is called a basic salt having a pH value of more than 7. Example – NaCN, Soap, NaF, Na2CO3, etc.
Also Read:-
And when a neutralization reaction carries out between a weak base and strong acid then the salt is formed which is called acidic salt having a pH value of less than 7. Example – NH4Cl, NH4NO3, NH4Br, etc.
Also Read:-
Also, if the case arises of the neutralization reaction between a strong acid and strong base then salt is formed which is called neutral salt having a pH value equal to 7. Example – NaNO3, NaBr, KNO3, KCl, KBr, etc.
Also Read:
- Why NaNO3 is a neutral salt?
- Why KBr is a neutral salt?
- Why KNO3 is a neutral salt?
- Why KCl is a neutral salt?
- Why NaBr is a neutral salt?
⇒ Strong base + Weak acid = Basic solution
⇒ Stronger acid + Weak base = Acidic solution
⇒ Strong acid + Strong base = Neutral solution
As we know, Sodium chloride is made from the neutralization reaction of strong acid (HCl) and strong base (NaOH).
Also Read:-
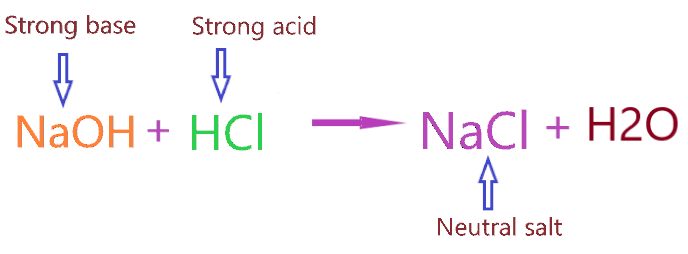
When strong acid and strong base reacts they tend to form the solution having a pH value equal to 7 i.e. the solution doesn’t have both acidic and alkaline properties.
Therefore, a solution of sodium chloride holds neither acidic properties nor alkaline properties.
Technically, Na+ and Cl– are very weak acids and bases or you can say both are of equal strength in an aqueous solution. Since they are very weak, both of them are not capable to undergone hydrolysis giving a neutral solution.
The conjugate acid of NaOH is Na+ which is too weak to function as an acid in water. The conjugate base of HCl is Cl– which is too weak to function as a base in water.
Using the ionic reaction of sodium chloride formation we can also understand why the aqueous solution of NaCl is considered neutral.
We know NaCl is formed when NaOH reacts with the HCl
⇒ NaOH + HCl → NaCl + H₂O
The ionic reaction of the above reaction can be written as-
⇒ Na+ + OH– + H+ + Cl– → Na+ + Cl– + H2O
Common ions from both(left and right sides) should be canceled out of each other.
Remaining ions we get-
⇒ OH– + H+ → H2O
∴ The final solution of NaCl contains an equal number of H+ and OH–, therefore, its aqueous solution is neutral.
Also Check:
The pH of the NaCl solution
The pH of a sodium chloride solution remains ≈7 due to the extremely weak basicity of the Cl− ion, which is the conjugate base of the strong acid HCl. In other words, NaCl has no effect on system pH in diluted solutions where the effects of ionic strength and activity coefficients are negligible. [Source]
Properties of Sodium chloride
- NaCl is an ionic compound with a 1:1 ratio of sodium and chloride ions.
- The molecular weight of sodium chloride is 58.44 g/mol.
- 100 g of NaCl contains 39.34 g Na and 60.66 g Cl.
- It has a boiling point of 1,465 °C and a melting point of 800.7 °C.
- It is easily soluble in water and appears as a white crystalline solid.
- It acts as a good conductor of electricity due to the free movement of ions in an aqueous solution.
- Sodium chloride is the most responsible for the salinity of seawater.
- It decomposes at high temperatures to produce toxic fumes of Na2O and HCl.
- About 1-5% of seawater is made of sodium chloride.
- At low concentrations, sodium chloride is non-toxic and non-hazardous.
Uses of Sodium chloride
- Sodium chloride is used in the soda ash industry.
- It is widely used in the production of glass.
- It is used as a food preservative and as a flavor enhancer in food industries.
- It is used in the Chlor-alkali process to produce chlorine, sodium hydroxide, and hydrogen.
- “Major application of sodium chloride is de-icing of roadways in sub-freezing weather.”
- In the metals extraction process, sodium chloride acts as an electrolyte and reduces the melting point of minerals.
- After the saponification process, sodium chloride is used to precipitate the soap.
- “It is used to increase the curing of concrete in cemented casings”.
- It is used as a cleansing agent rubbed on household surfaces.
- It is used in the production of sodium sulfate and hydrochloric acid.
To quickly recap, here are some reasons why NaCl is neither acid nor base:
- NaCl doesn’t release H+ ions in an aqueous solution, hence, it is not acid.
- For same reason, NaCl doesn’t release OH– ions in an aqueous solution, hence, it is not base.
- NaCl is neutral salt, made from the neutralization of strong acid (HCl) with a strong base (NaOH).
Summary
Sodium chloride is an ionic compound made up of two ions(Na+ and Cl–). It is the most common salt, we use in our everyday life. It is commonly used as a food preservative. This salt is very important for multicellular organisms. At last, we will take an overview of this article on “Is NaCl an acid or base or salt?”
- The pH value of the sodium chloride solution is 7.
- Is Sodium chloride(NaCl) an acid or base? NaCl is a neutral salt. It is made from the neutralization reaction carried out between the strong acid, namely Hydrochloric acid (HCl), and the strong base, namely Sodium hydroxide (NaOH).
- Sodium chloride doesn’t hold the properties of acidic and alkaline in an aqueous solution.
- The aqueous solution of NaCl is neutral. Because it contains an equal number of H+ and OH– ions, so both have the same strength, hence, neither of them is able to alter the pH of the final solution.
- NaCl is salt in nature because when NaCl is dissolved in an aqueous solution it breaks apart into two ions i.e. Na+ and Cl–. These ions are extremely weak acid and base in nature so neither of them is capable of hydrolyzing. As a result, the pH of an aqueous solution containing the ions of NaCl remains neutral.
About the author
Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. The team at Topblogtenz includes experts like experienced researchers, professors, and educators, with the goal of making complex subjects like chemistry accessible and understandable for all. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/

Very informative, excellent
Thanks!
So, I came on here to try and debunk possible myths that adding salt to coffee will neutralize its acidity. According to this, because salt cannot modify protons of hydrogen (not having any itself), it cannot affect the pH of water?