Is Sodium bromide (NaBr) an acid or base or salt?

Sodium bromide is an inorganic compound that appears as a white powder and has a chemical formula NaBr. It produces bromine gas when heated very strongly in the air. The crystalline structure of sodium bromide resembles sodium chloride.
In this article, we will discuss Is Sodium bromide (NaBr) an acid or base or neutral?
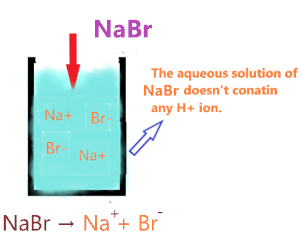
So, Is NaBr an acid or base? NaBr is neither an acid nor base, it is a neutral salt as it is made from the neutralization reaction of the strong acid(HBr) with a strong base(NaOH). The pH value of the aqueous solution of NaBr is 7. Because strong acid and a strong base will neutralize each other effects and a neutral solution forms.
| Name of Molecule | Sodium bromide |
| Chemical formula | NaBr |
| Molar mass | 102.8 g/mol |
| Nature | Neither acid nor base |
| pH value | 7 |
Why NaBr can't be acid in nature?
A compound is said to be acid when it is capable of releasing the proton or H+ ion when dissolved in an aqueous solution. According to Arrhenius’s theory of acid ” A compound is said to be acid when added in water, it increases the number of H+ ions in solution.
Since NaBr doesn’t contain any hydrogen ion, it is unable to release the proton in an aqueous solution.
So, as per Arrhenius’s theory, NaBr aqueous solution doesn’t have any property of acid due to the absence of H+ ions. Therefore, we can say NaBr is not an Arrhenius acid.
Bronsted-Lowry’s theory for an acid state that ” A compound is said to be acid when dissolved in water they donate the proton to other species and make conjugate base”.
Therefore, once again NaBr doesn’t have any proton, so obvious it is not able to donate the proton to other species.
Hence, NaBr is also not a Bronsted-Lowry acid.
Why NaBr can't be base in nature?
I am sure you are aware of the term “Base” we frequently use in chemistry. “A base is any substance that increases the concentration of OH– ion in aqueous solution”.
NaBr is not a base because it doesn’t have any OH– ion to liberate when dissolved in an aqueous solution.
According to the Bronsted-Lowry theory for base ” A compound is said to be base when it accepts the proton from other species and forms a conjugate acid”.
One might argue that NaBr is a Bronsted-Lowry base because one of its ions (Br–) accepts the proton when dissolved in water and forms a conjugate acid(HBr).

However, this is not the case, Na+ and Br– is very very weak acid and base, you can simply treat them as neutral as they don’t have any observable effect on the pH of an aqueous solution.
Br– is a very weak conjugate base of the strong acid(HBr), hence, it will not react with the either of the H2O ions.
In short, Na+ and Br– ions are just spectator ions in the solution since they doesn’t take part in chemical reaction.
Therefore, we can say, NaBr is also not a Bronsted-Lowry base as neither of its ions is capable of accepting the proton.
Why NaBr is neutral salt?
“A salt is an ionic compound that contains a cation (base) and an anion (acid).”
According to the concept of salt, when a neutralization reaction carries out between a strong base and weak acid then the salt is formed which is called a basic salt having a pH value of more than 7. Examples – NaCN, Soap, NaF, Na2CO3, etc.
Also Read:-
And when a neutralization reaction carries out between a weak base and strong acid then the salt is formed which is called acidic salt having a pH value of less than 7. Example – NH4Cl, NH4NO3, NH4Br, etc.
Also Read:-
Also, if the case arises of the neutralization reaction between a strong acid and strong base then salt is formed which is called neutral salt having a pH value equal to 7. Example – NaNO3, NaCl, KNO3, KCl, KBr, etc.
Also Read:
- Why NaCl is a neutral salt?
- Why NaNO3 is a neutral salt?
- Why KBr is a neutral salt?
- Why KNO3 is a neutral salt?
- Why KCl is a neutral salt?
⇒ Strong base + Weak acid = Basic solution
⇒ Stronger acid + Weak base = Acidic solution
⇒ Strong acid + Strong base = Neutral solution
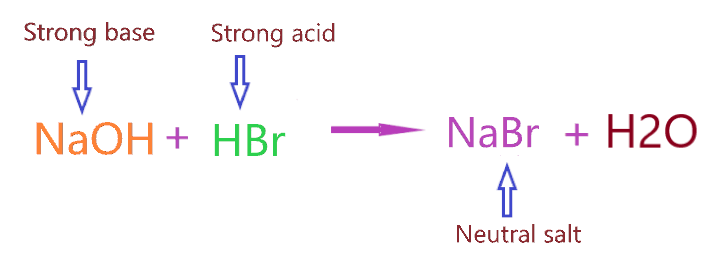
As we know, Sodium bromide is made from the neutralization reaction of strong acid(HBr) and strong base(NaOH).
Also Read:-
When strong acid and strong base reacts they tend to form the solution having a pH value equal to 7 i.e. the solution doesn’t have both acidic and alkaline properties.
Therefore, a solution of sodium bromide holds neither acidic properties nor alkaline properties.
Technically, Na+ and Br– are very weak acids and bases or you can say both are of equal strength in an aqueous solution. Since they are very weak, both of them are not capable to undergone hydrolysis giving a neutral solution.
Using the ionic reaction of sodium bromide formation we can also understand why the aqueous solution of NaBr is considered neutral.
We know NaBr is formed when NaOH reacts with the HBr.
⇒ NaOH + HBr → NaBr + H₂O
The ionic reaction of the above reaction can be written as-
⇒ Na+ + OH– + H+ + Br– → Na+ + Br– + H2O
Common ions from both(left and right sides) should be canceled out of each other.
Remaining ions we get-
⇒ OH– + H+ → H2O
∴ The final solution of NaBr contains an equal number of H+ and OH–, therefore, its aqueous solution is neutral.
Also Check:
Properties of Sodium bromide
- It has a boiling point of 1,390 °C and a melting point of 747 °C.
- It is soluble in liquid, ammonia, alcohol, etc. but insoluble in acetone and acetonitrile.
- It has a cubic crystal structure.
- It has no odor.
- It is readily soluble in water.
- It does not occur as a natural solid due to its solubility.
Uses of Sodium bromide
- It is used to prepare materials for oil wells such as dense drilling fluids.
- It is used as a disinfectant for swimming pools and hot tubs.
- It is used to prepare bromides ion in organic synthesis.
- It is used in the medical industry as an anticonvulsant and a sedative.
- It is also used as a reagent in pharmaceutical preparations.
Summary
Sodium bromide appears as a white crystalline solid with a salty or bitter taste. It is prepared by treating hydrogen bromide with sodium hydroxide. It is an ionic compound in nature. Inhalation of a large amount of sodium bromide can cause many health hazards such as nausea, headache, dizziness, etc.
- The pH value of the sodium bromide solution is 7.
- Is Sodium bromide(NaBr) an acid or base or salt? NaBr is a neutral salt. It is made from the neutralization reaction carried out between the strong acid, namely Hydrobromic acid (HBr), and strong base, namely Sodium hydroxide (NaOH).
- Sodium bromide doesn’t hold the properties of acidic and alkaline in an aqueous solution.
- The aqueous solution of NaBr is neutral. Because it contains an equal number of H+ and OH– ions, so, both have the same strength, hence, neither of them is able to alter the pH of the final solution.
- NaBr is salt in nature because when NaBr is dissolved in an aqueous solution it breaks apart into two ions i.e. Na+ and Br–. These ions are extremely weak acid and base in nature so that neither of them is capable of hydrolyzing. As a result, the pH of an aqueous solution containing the ions of NaBr remains neutral.
About the author
Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. The team at Topblogtenz includes experts like experienced researchers, professors, and educators, with the goal of making complex subjects like chemistry accessible and understandable for all. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/

