Is Sodium carbonate (Na2CO3) an acidic or basic or neutral? Strong or Weak

Sodium carbonate is an inorganic compound composed of sodium salt and a carbonate salt having the chemical formula Na2CO3. It is commonly known as washing soda or soda ash. It appears as a white solid and soluble in water. It mostly used as a cleansing agent for domestic purposes.
In this article, we will discuss Is Sodium carbonate (Na2CO3) an acid or base or neutral salt?
So, Is Na2CO3 an acid or base? Na2CO3 is a basic salt. It is formed from the neutralization of a Strong base, namely Sodium hydroxide (NaOH), and Weak acid, namely Carbonic acid (H2CO3). The pH value of the aqueous solution of sodium carbonate is greater than 7.
Let’s dive into more detail about why Na2CO3 acts as basic salt?
| Name of Molecule | Sodium carbonate |
| Chemical formula | Na2CO3 |
| Nature | Basic salt |
| Molar mass | 105.9888 g/mol |
| pH | > 7 |
Why Sodium carbonate(Na2CO3) is basic salt in nature?
Have you ever heard what do these terms mean by “Acidic salt”, “Basic salt”, “Neutral salt”, “Amphoteric salt”?
Acidic salt: Acidic salt is formed when a neutralization reaction carries out between strong acid and weak base. This type of salt produces an acidic solution after being dissolved in a solvent that has a pH value of less than 7. Examples of acidic salt – NH4Cl, NH4NO3, NH4Br, etc.
Also Read:-
Basic salt: Basic salt is formed when a neutralization reaction carries out between a strong base and weak acid. This type of salt produces a basic solution after being dissolved in a solvent that has a pH value of more than 7. Examples of basic salt – are NaCN, Soap, NaF, etc.
Also Read:-
Neutral salt: This salt has a pH value equal to 7 which means it neither has acidic properties nor basic properties. It is formed when a neutralization reaction carries out between a strong acid and strong base or weak acid and weak base. Examples of neutral salt – are NaCl, KCl, NaNO3, etc.
Also Read:-
Amphoteric salt: This salt can act as acidic salt as well as a basic salt. Or “Amphoteric means the compound has the ability to act as an acid with a base and base with an acid”. Examples of Amphoteric salts – NaHCO3, HCO3–, CH3OH, etc.
Also Read:-
- Why does NaHCO3 act as acid as well as the base?
- Why does HCO3- act as acid as well as the base?
- Why does CH3OH act as acid as well as a base?
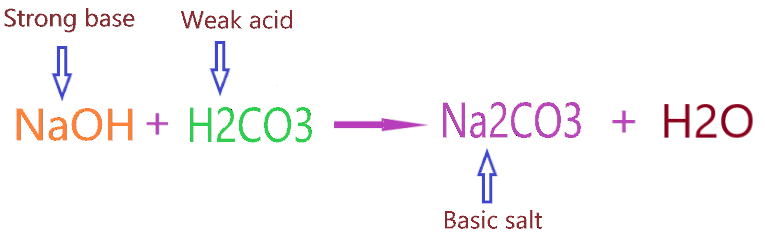
Ok, Now come to the point Why does Na2CO3 act as basic salt? As we know, Sodium carbonate is formed when NaOH(Strong base) reacts with H2CO3(Weak acid). They react with each other in the molar ratio of 2:1.
⇒ 2NaOH + H2CO3 → Na2CO3 + 2H2O
As per the concepts of salts, a combination of a strong base and weak acid leads to the formation of basic salt.
The nature of salt(acidic or basic) is dependent on the strength of the component in the acid-base reaction.
- An aqueous solution of stronger acid and weak base will acquire more property of acidic instead of a base.
- An aqueous solution of a stronger base and weak acid will acquire more property of basic instead of an acid.
In the case of Na2CO3, a stronger base (NaOH) reacts with a weaker acid (H2CO3), so, the aqueous solution of its acquires more property of base rather than an acid.
Hence, we can say Na2CO3 is a basic salt in nature.
Why the aqueous solution of Na2CO3 becomes basic?
An aqueous solution having a pH value of more than 7 is called a basic aqueous solution.
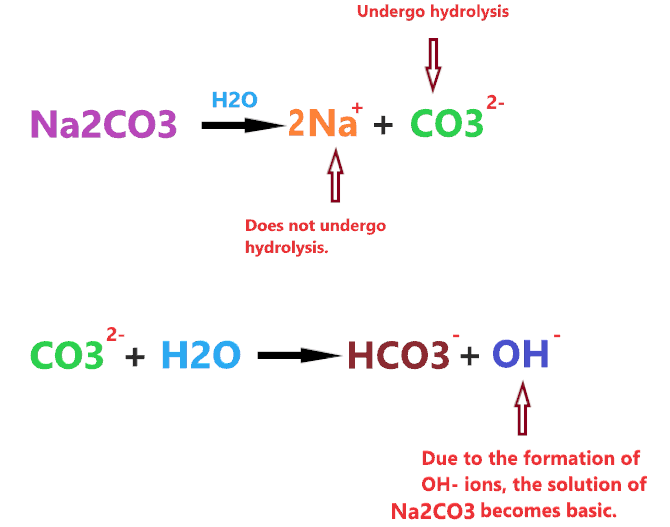
When sodium carbonate is dissolved in water, it dissociates into two ions Na+ and CO32-.
⇒ Na2CO3 → 2Na+ + CO32-
The Na+ is a cation of strong base(NaOH), hence, it will not undergo hydrolysis in water and doesn’t affect the pH of the solution. But the CO32- is the anion of a weak acid that gets hydrolysis in water to form bicarbonate ions(HCO3–), leaving OH– ions that make the solution basic.
⇒ CO32- + H2O → HCO3– + OH–
The bicarbonate ion(HCO3–) remains mostly unionized in the solution, hence, the final solution contains more OH– ion than H+ ion.
Therefore, with the presence of additional OH– ions in the aqueous solution, sodium carbonate (Na2CO3) solution becomes basic in nature.
Note: An aqueous solution nature depends on the H+ and OH– ions. When more OH– ions are present in an aqueous solution, then the solution becomes basic, and when more H+ ions are present in an aqueous solution, the solution becomes acidic.
Also, when the number of H+ and OH– ions same in an aqueous solution, then the solution is said to be neutral.
Why Na2CO3 is not acidic or neutral salt?
A neutral salt means showing no effect of acidic or alkaline properties when dissolved in water and an acidic salt means having more properties of acid when dissolved in an aqueous solution with a pH value of less than 7.
The important concept of acid-base strength–
- A stronger base + weaker acid forms a basic aqueous solution due to the presence of more OH– ions. (OH– > H+)
- Stronger acid + weaker base forms an acidic aqueous solution due to the presence of more H+ ions. (H+ > OH–)
- Stronger/weaker acid + Stronger/weaker base forms a neutral aqueous solution due to the presence of the same number of OH– and H+ in the solution. (H+ = OH–)
So, Why is sodium carbonate not acidic or neutral salt in nature? This is because when Na2CO3 is dissolved in an aqueous solution it will dissociate into 2Na+ cation and CO32- anion. The cation(Na+) is useless since it doesn’t take part in a chemical reaction because of its very weak nature.
Whereas the anion(CO32-) reacts with H+ and forms a respective acid(HCO3–), leaving OH– ions in the solution.
⇒ CO32- + H+OH– → HCO3– + OH–
So, the aqueous solution of Na2CO3 contains a large number of OH– ions due to the hydrolysis of CO32- ions.
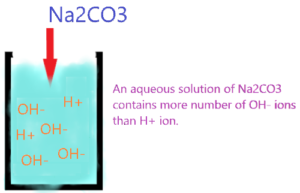
As shown in the figure, the aqueous solution of Na2CO3 contains a large number of OH– ions than H+ ions, this results in an increase in the pH of the solution, which causes Na2CO3 to become alkaline.
Therefore, Na2CO3 is not acidic or neutral salt due to the presence of more OH– ions that strongly indicate that it will hold the nature of the base.
Note: Sodium carbonate(Na2CO3) is not a base, it is basic salt and an aqueous solution of it holds basic nature.
Also read:–
Is Na2CO3 considered strong or weak(acidic or basic salt)?
Sodium carbonate is neither acidic nor basic, it is salt made up of a strong base and weak acid. Strong bases dissociate completely in solution and release a large number of OH– ions but the weak acid mostly remains undissociated and releases fewer H+ ions.
Therefore, with the presence of more OH– ions in an aqueous solution of Na2CO3, we can consider it strong basic salt.
The concept of acidic salt and basic salt:-
⇒ Strong base + Weak acid = Basic salt (∴ OH– ions > H+ ions)
⇒ Stronger acid + Weak base = Acidic salt (∴ H+ ions > OH– ions)
Also Check:
Uses of Sodium carbonate
- Sodium carbonate is used for making glass and paper.
- It is used to remove the permanent and temporary hardness of the water or you can say it is used to soften the water.
- It is used in household work as a cleansing agent for washing clothes.
- It is used to regulate acidity and maintain alkaline conditions in various chemical processes.
- Sodium carbonate is also used in medicine to treat ringworm, cleanse the skin, and treat eczema.
- It is used as a foaming agent in toothpaste and as a wetting agent in the brick industry.
Properties of Sodium carbonate
- It has octahedral coordinate geometry.
- It has a boiling point of 1,600 °C and a melting point of 851 °C.
- It is preferred over sodium hydroxide due to its non-corrosive nature.
- It is soluble in water and glycerol but insoluble in acetone, alcohol, etc.
- The acidity (pKa) value of sodium carbonate is 10.33.
Summary
Sodium carbonate is one of the common compounds we used in our home for purposes like washing clothes, also known as washing soda or soda ash. It is safer to handle that’s why it is preferred over sodium hydroxide in various chemical processes. At last, we will overview this article on “Is Na2CO3 an acid or base or neutral salt?”
- Is sodium carbonate (Na2CO3) acidic or basic salt? Na2CO3 is a basic salt having a pH value close to 11, made from the neutralization of a strong base(NaOH) with a weak acid (H2CO3).
- The aqueous solution of sodium carbonate(Na2CO3) is basic in nature due to having more hydroxide ions produced from the hydrolysis of carbonate ions (CO32- + H2O → HCO3– + OH–).
- The presence of fewer hydrogen ions and more OH– ions, increases the pH level of the Na2CO3 solution.
About the author
Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. The team at Topblogtenz includes experts like experienced researchers, professors, and educators, with the goal of making complex subjects like chemistry accessible and understandable for all. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/

