How to know/Determine if Base or Acid is Strong or Weak?

Many of you must be confused about “How to know if the acid or base is strong or weak”? Is there any formula for determining the strength of acid or base?
Well, It is very easy to know whether given acid or base is strong or weak in nature if you are aware of their basic concept.
The best way to tell or determine whether acid or base is strong or weak to look at following condition-
- Any acid that dissociates 100% into ions is called a strong acid. If it does not dissociate 100%, it is a weak acid.
- A strong base is a base that is 100% ionized in solution. If it is less than 100% ionized in solution, it is a weak base.
First of all, there are only a few strong acids and bases in chemistry, so, memorize the list of strong ones and consider the remaining ones weak in nature.
List of strong acids
- HNO3 – Nitric acid
- HCl – Hydrochloric acid
- HI – Hydroiodic acid
- HClO4 – Perchloric acid
- HBr – Hydrobromic acid
- HClO3 – Chloric acid
- H2SO4 – Sulfuric acid
That’s it, there are only 7 common strong acids in chemistry, hence, commit these strong acids to your memory.
For example – if you are given a compound named such as nitrous acid, formic acid, acetic acid, etc. you should remember the list of strong acids and mark them as weak acid in nature as they are not part of the strong acid list.
List of strong bases
- NaOH – Sodium hydroxide
- KOH – Potassium hydroxide
- LiOH – Lithium hydroxide
- CsOH – Cesium hydroxide
- RbOH – Rubidium hydroxide
- Ca(OH)2 – Calcium hydroxide
- Ba(OH)2 – Barium hydroxide
- Sr(OH)2 – Strontium hydroxide
So, there are 8 common strong bases in chemistry, and any base that is not listed here is considered a weak base in nature.
Therefore, one of the easiest ways to know whether acid/base is strong or weak, go through the list of strong acid and base.
Now we will discuss all the concepts regarding the strength of acid and bases.
Let’s start with the strength of the acidic compounds.
How to tell if given acid is strong acid?
The strength of an acid is determined by the H+ ion concentration in its aqueous solution.
For knowing whether an acid is a strong acid or not, look out the basic definition of strong acid
Strong acid: A strong acid is a compound that is 100% ionized or completely dissociates in an aqueous solution and gives a high amount of hydrogen ions. It means all the moles of acid will dissociate to yield H+ ions and no undissociated moles of acid remains in the solution.
Characteristics of strong acids
- They have a low pH value around 0 to 1.
- The higher the Ka or lower the pKa, the stronger the acid.
- They have weak bond strength, hence, dissociate easily into a large number of ions.
- The stronger an acid is, the more easily it loses a proton, H+ ions.
As we know, HCl is a strong acid, let’s see what happens when we dissolve it in an aqueous solution.
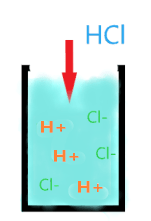
When HCl is dissolved in an aqueous solution, it is completed ionized into the two ions(H+ and Cl–) and liberates a large number of H+ ions. So, if you dissolved the 10 moles of HCl or 20 moles, it does not matter, all these moles will completely dissociate into the ions.
As when 10 moles of HCl are dissolved in water, then, it will yield 10 moles of the H+ ion and 10 moles of the Cl– ion. None of the non-ionized HCl remains in the solution, that’s why it is a strong acid.
⇒ HCl(aq) → H+(aq) + Cl–(aq)
The single arrow in the above reaction shows that only ions(H+ and Cl–) of the acids are present in the solution, none of the HCl molecules remains in the solution.
So, that’s a simple example of a strong acid, you can define all remaining strong acids such as HNO3, HBr, HClO4, etc. according to the above explanations.
The stronger acids have high acid dissociation constant value and a smaller logarithmic constant(pKa).
The acid dissociation constant (Ka) is a quantitative measure of the strength of an acid in solution.
⇒ If the value of the dissociation constant of acid is way greater than 1 (Ka >> 1), then the nature of the compound is a strong acid.
⇒ “The lower the value of pKa, the stronger the acid and the greater its ability to donate its protons.”
The list of strong acids with their Ka and pKa value.
| Name | Chemical Formula | Ka value | pKa value |
| Nitric acid | HNO3 | 2.3 × 101 | -1.37 |
| Hydrochloric acid | HCl | 1.3 × 106 | -6.3 |
| Hydroiodic acid | HI | 2 × 109 | -9.3 |
| Perchloric acid | HClO4 | >>1 | -8 |
| Hydrobromic acid | HBr | 1 × 109 | -8.7 |
| Sulfuric acid | H2SO4 | 1 × 102 | -9.3 |
So, you can easily find whether the given acid is strong or not, also, we already discussed there are only a few common strong acids in chemistry, hence, memorizing them will greatly help you.
If you want to read in detail about some molecules, why they are strong acids and not weak, please go through the links given below.
How to determine a weak acid?
For determining whether an acid is a weak acid or not, look out for the basic definition of a weak acid.
A weak acid is a compound that partially dissociates, which means, that not all molecules of weak acid dissociate in an aqueous solution to yield an H+ ion, or, they are not 100 % ionized in an aqueous solution.
Characteristics of weak acids
- They have a pH value of around 3 to 7.
- The lower the Ka or higher the pKa, the weaker the acid.
- They have high bond strength, hence, don’t dissociate easily in an aqueous solution.
- The weaker acid is, the more difficult it is to lose the H+ ions.
At equilibrium, both undissociated acids and their ionized product are present in the solution.
Let’s take an example of acetic acid which is a weak acid because it is not in the list of some common strong acids.
Let’s see what happens when CH3COOH as a weak acid dissolved in an aqueous solution.

So, when CH3COOH is dissolved in water, then, it doesn’t dissociate completely into the ions, it only partially dissociates and liberates only a few H+ ions in the solution. Most of the CH3COOH remains undissociated in the solution, only a few of them dissociated to give H+ ions, hence, it is a weak acid.
It means if you dissolve the 10 moles of CH3COOH in an aqueous solution, then, only a few moles of it will dissociate completely into the ions, maybe from 10 moles, only 2 dissociate or 3 or 5 dissociate but not all 10 moles of CH3COOH will dissociate, that why it is a weak acid because of partial dissociation.
⇒ CH3COOH(aq) ⇆ H+(aq) + CH3COO–(aq)
The double arrow in the above reaction shows that the reaction will proceed in both directions at equilibrium but the backward reaction is more favorable than the forwarding reaction which means the splitting ions(H+ + CH3COO–) easily react with each other to reform the CH3COOH molecule.
So, that’s a simple example of a weak acid, you can define all remaining weak acids such as HCN, HF, HCOOH, etc. according to the above explanations.
The weaker acids have a low acid dissociation constant value and a larger logarithmic constant(pKa).
⇒ If the value of the dissociation constant of acid is way lower than 1 (Ka << 1), then the nature of the compound is a weak acid.
The list of some important weak acids with their Ka and pKa value.
| Name | Chemical formula | Ka value | pKa value |
| Hydrocyanic acid | HCN | 6.2 × 10-10 | 9.21 |
| Phosphoric acid | H3PO4 | 7.1 × 10-3 | 2.16 |
| Hydrofluoric acid | HF | 6.3 × 10-4 | 3.20 |
| Nitrous acid | HNO2 | 5.6 × 10-4 | 3.25 |
| Formic acid | HCOOH | 1.78 × 10-4 | 3.75 |
| Carbonic acid | H2CO3 | 4.4 × 10-7 | 6.35 |
| Acetic acid | CH3COOH | 1.7 × 10-5 | 4.76 |
| Ammonium ion | NH4+ | 5.6 × 10-10 | 9.25 |
Also read:-
How to know if given base is strong base?
The strength of a base is determined by the OH– ion concentration in its aqueous solution.
For knowing whether a base is a strong base or not, look out for the basic definition of a strong base.
Strong base: A compound is a strong base when it completely dissociates in an aqueous solution and liberates a large number of hydroxide ions. All moles of the strong base dissociate into hydroxide ions(OH–) and no moles of it remain undissociated in the solution.
Characteristics of a strong base:
- They have a high pH value of around 10 to 14.
- The higher the Kb or lower the pKb, the stronger the base.
- The stronger base is more capable of accepting a proton from other compounds.
- They are good electrolytes and highly reactive in nature.
Almost all strong bases contain OH– ions and they are 100% ionized in an aqueous solution.
As we know, NaOH is a strong base, let’s see what happens when we dissolve it in an aqueous solution.

So, when NaOH is dissolved in water, it is completely ionized into the ions(Na+ and OH–). No undissociated molecule(NaOH) is present in the solution, only ionized ions are present everywhere in the solution.
The single arrow used in the above reaction shows that only forward reaction takes place at equilibrium and no backward reaction occurs in the solution.
Hence, you can also define other strong bases such as KOH, LiOH, Ca(OH)2, etc as per the above explanations.
The stronger base has a high base dissociation constant value and a smaller logarithmic constant(pKb).
The base dissociation constant (Kb) is a quantitative measure of the strength of an base in solution.
⇒ If the value of the dissociation constant of the base is way greater than 1 (Kb >> 1), then the nature of the compound is a strong base.
The list of important strong bases with their Kb and pKb value.
| Name | Chemical formula | Kb value | pKb value |
| Sodium hydroxide | NaOH | >1 | <1 |
| Potassium hydroxide | KOH | >1 | <1 |
| Lithium hydroxide | LiOH | >1 | <1 |
| Calcium hydroxide | Ca(OH)2 | >1 | <1 |
| Barium hydroxide | Ba(OH)2 | >1 | <1 |
Also read:-
How to know if given base is a weak base?
For knowing whether a base is a weak base or not, look out for the basic definition of a weak base.
Weak base: A compound is a weak base when it partially or not completely dissociate in an aqueous solution which means not all moles of the base dissociate in a solution to yield OH– ion, and at equilibrium, both undissociated base and their ionized product present in the solution.
Characteristics of a weak base:
- They have a low pH value of around 7 to 10.
- The lower the Kb or higher the pKb, the weaker the base.
- The weaker base is less capable of accepting a proton from other compounds.
- They are less reactive as compared to a strong base.
The best way to distinguish a weak base from a strong base is that-
“All strong bases are OH– compounds. So a base based on some other mechanism, such as NH3 (which does not contain OH− ions as part of its formula), will be a weak base.”
Some examples of the weak bases are – NH3, CH3NH2, C5H5N, etc.
These compounds are proton acceptors in water, therefore, considered as a base but they don’t contain OH compound, hence, they are defined as a weak base.
Let’s take a look at the aqueous solution of the CH3NH2 compound.

When CH3NH2 is dissolved in water, it accepts the hydrogen ion from the water molecule and produces two ions(CH3NH3+ and OH–) but the ion(CH3NH3+) is not stable in an alkaline environment, it keeps breaking into CH3NH2 and H+.
Hence, not all the CH3NH2 molecules react with water ions and produce OH– ions, most of them stay together, only, a few molecules do interact with water, therefore, CH3NH2 is considered a weak base in nature.
The double arrow in the reaction of methylamine and water shows that both forward and backward reactions occur at equilibrium. But the backward reaction will dominate the solution.
The weaker base has a low base dissociation constant value and a larger logarithmic constant(pKb).
⇒ If the value of the dissociation constant of the base is way lower than 1 (Kb << 1), then the nature of the compound is a weak base.
The list of important weak bases with their Kb and pKb value.
| Name | Chemical formula | Kb value | pKb value |
| Methylamine | CH3NH2 | 4.6 × 10-4 | 3.34 |
| Ammonia | NH3 | 1.8 × 10-5 | 4.75 |
| Pyridine | C5H5N | 1.7 × 10-9 | 8.77 |
| Aniline | C6H5NH2 | 7.4 × 10-10 | 9.13 |
Also read:-
Is there any formula for identify the strong or weak acid/base?
There is no direct formula or hard rule for determining the strength of acid or base. The best way to predict the strength of an acid or base is to memorize the shortlist of common strong acids or bases and considered the rest are weak or remember their dissociation constant value if you can.
The list of some common strong or weak acids/bases.
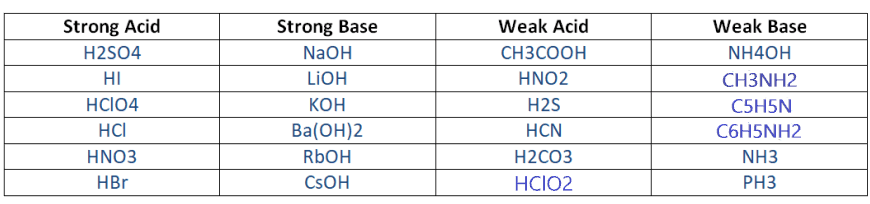
Important: In this article, we discuss the strength of acid and base relative to the aqueous solution that has a pH close to 7. You cannot define any acid or base as absolute strong or weak in nature. They all vary with the relative compound.
As we know, H2SO4 is a really strong acid but when it reacts with superacids like CF3SO3H and HSO3F, then, H2SO4 would be considered as a very weak acid as compared to superacid strength.
But, for now, you can ignore this part and only concentrate on the strength of acid and base relative to the water solution.
Also check:- How to tell if something is acid or base or salt or neutral?
Summary
- The best way to tell/determine if the base or acid is strong or weak is to memorize the list of strong acid and base only and consider the remaining ones weak in nature.
- There are 7 common strong acids – HCl, HBr, HNO3, H2SO4, HI, HClO3, HClO4, and 8 common strong bases – NaOH, KOH, CsOH, RbOH, LiOH, Ba(OH)2, Ca(OH)2, Sr(OH)2.
- Strong acid and bases are completely ionized in a solution, none of the non-ionized molecules are left in the solution, and all are dissociated into the ions.
- Weak acid and bases are only partially ionized in solution, most of the molecules remain undissociated in solution.
- Stronger acids have a larger acid dissociation constant value and lower pKa value whereas weaker acids have a smaller acid dissociation constant value and higher pKa value.
- Weaker acids form a stronger conjugate base and stronger acids form a weaker conjugate base.
- Stronger bases form a weaker conjugate acid and vice-versa.
Note: Please go through the links in this article, if you find difficulty in understanding why the given compound is strong or weak.
About the author
Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. The team at Topblogtenz includes experts like experienced researchers, professors, and educators, with the goal of making complex subjects like chemistry accessible and understandable for all. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/
