Is Lithium hydroxide (LiOH) an acid or base? - Strong or Weak

Lithium hydroxide is an inorganic compound with the chemical formula LiOH. It appears as a white solid and is generally odorless in nature. It is easily soluble in water and mainly consumed in the production of cathode materials for lithium-ion batteries.
In this article, we will discuss Is Lithium hydroxide (LiOH) an acid or base? Is it strong or weak? etc.
So, Is LiOH an acid or base? LiOH is a base. Because when it is dissolved in an aqueous solution, it gives two ions Li+ and OH–. And any molecule which gives OH– after dissolving in water is defined as a base in nature. It has a pH value greater than 7.
| Name of Molecule | Lithium hydroxide |
| Chemical formula | LiOH |
| pH value | >7 |
| Conjugate acid | Li+ |
| Nature | Strong base |
| Basicity (pKb) | -0.04 |
Why LiOH is base?
A base is defined as a proton acceptor or lone pair donor. When LiOH dissolves in water is split into two ions Li+ and OH–.
As LiOH dissociates into Li+ and OH–, this OH– ion accepts the proton (H+) to become water.
⇒ LiOH + (aq) → Li+ (aq) + OH– (aq)
Theoretically, we have three concepts to check whether LiOH is acid or base.
(a). Arrhenius concept
(b). Bronsted-Lowry concept
(c). Lewis concept
(1). Arrhenius concept:
According to the Arrhenius concept, the compound is said to be base when it produces OH– ions through ionization or through dissociation in water and increases the concentration of OH– ions in an aqueous solution.
Clearly, when LiOH is dissolved in an aqueous solution it produces OH– ions, hence, LiOH is Arrhenius base.
(2). Bronsted-Lowry concept:
According to the Bronsted-Lowry concept, a compound is said to be base when it accepts the proton from other compounds. Or you can say proton acceptor is said to be Bronsted-Lowry base.
Take an example for knowing whether LiOH base or acid according to the Bronsted-lowry concept-
Consider the reaction of LiOH with HCl.
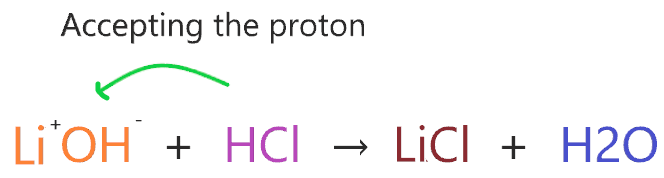
According to the above reaction, LiOH has two ions Li+ and OH–, when they react with HCl then OH– accepts H+ from HCl to forms H2O.
So, LiOH ion accepting the proton from another compound, and according to this theory, LiOH is a Bronsted-Lowry base.
(3). Lewis concept:
Here comes the most important concept of acid and base. According to the lewis theory, a compound is said to be acid when it accepts the pair of electrons and a compound is said to be base when it donates the pair of electrons.
What does it mean?
It means Lewis acid is a compound that has a deficiency of electrons that’s why they are electron-pair acceptors and lewis’s base is a compound that is rich in electrons so, they are electron-pair donors.
⇒ Lewis acid → electron pair acceptor
⇒ Lewis base → electron pair donator
As LiOH contains OH– ion that has three lone pairs of electrons and it can easily donate these pairs of electrons to form a covalent bond with another compound acceptor.
So, we can say LiOH is lewis’s base also.
Is LiOH a strong base or weak base?
To know whether LiOH is a strong base or a weak base, you must know the basic difference between a strong base or a weak base.
Strong base: A compound is a strong base when it completely dissociates in an aqueous solution and liberates a large number of hydroxide ions. All moles of the base dissociates to yield hydroxide ion(OH–) and no undissociated base should be left in the solution. Example – NaOH, KOH, Ba(OH)2, Ca(OH)2, etc.
Also Read –
- Is NaOH a strong or weak base?
- Is KOH a strong or weak base?
- Is Ba(OH)2 a strong or weak base?
- Is Ca(OH)2 a strong or weak base?
Weak base: A compound is a weak base when it partially or not completely dissociates in an aqueous solution. It means only some parts of the weak base dissociate in the solution to give OH– ion but some parts remain undissociated inside the solution. Example- NH3, CH3NH2, NH4OH, etc.
Also Read –
So, Is Lithium hydroxide (LiOH) a strong base or a weak base? LiOH is the strong base because it completely dissociates in an aqueous solution to give OH– ion and no moles of it remain undissociated inside the solution. And the amount of OH– ions in an aqueous solution is very high and we know OH– ions have a tendency to accept the proton.
So, more proton acceptors present in the solution ultimately make the LiOH a strong base.
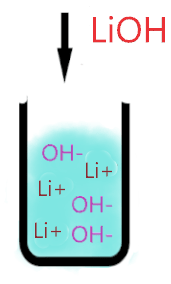
As you see in the above aqueous solution when LiOH is dissolved in water, it is completely ionized into the ions(Li+ and OH–). No undissociated molecule(LiOH) is present in the solution, only ionized ions are present everywhere in the solution.
⇒ LiOH + (aq) → Li+ (aq) + OH– (aq)
The single arrow used in the above reaction shows that only forward reaction takes place at equilibrium and no backward reaction occurs in the solution.
As Li+ is a very weak conjugate acid of LiOH, hence it has no ability to react with either OH– ion or with water molecules ions. Thus, only splitting ions(Li+ and OH–) remain in the solution.
Or you can also assume the Li+ as a spectator ion because it is almost useless in solution, it has no effect on the pH value of the solution.
“A spectator ion is an ion that does not take part in the chemical reaction and is found in solution both before and after the reaction.”
Hence, a large number of hydroxide ions present in the aqueous solution of LiOH, steadily increase the pH value and rises the effect of the basic in solution.
Also, the base dissociation constant value(Kb) for LiOH is greater than 1.
An base dissociation constant (Kb) is a quantitative measure of the strength of an base in solution.
⇒ If the value of the dissociation constant of the base is greater than 1 (Kb > 1), then the nature of the compound is a strong base.
⇒ If Kb < 1, then the nature of the compound is a weak base.
So, the higher the value of the base dissociation constant, the larger the strength of a base in solution.
Here’s the list of some common strong/weak acids and bases.

Also check:-
Why LiOH is not an acid?
Acid is a compound that donates H+ ions when dissolving them in an aqueous solution. It means the acid is the proton donator.

So, Is LiOH is an acid? LiOH is not an acid because when it dissolves in an aqueous solution, it furnishes a large amount of OH– ions. And OH- ion is a good acceptor of the proton, which means LiOH ions are proton acceptors, not proton donators.
⇒ LiOH + (aq) → Li+ (aq) + OH– (aq)
We have two theories given by Arrhenius and Bronsted-Lowry to check why LiOH is not acid?
⇒ According to the Arrhenius theory, any compound is said to be acid when it liberates H+ ion in water or aqueous solution. And we know LiOH in an aqueous solution only produces OH– ion. So, LiOH is not an Arrhenius acid.
⇒ According to the Bronsted-lowry theory, any compound is said to be acid when it has a tendency to donate the proton to other compounds. Let’s take an example to understand this-
⇒ LiOH(aq) + HCl(aq) → LiCl(aq) +H2O(l)
When LiOH reacts with a strong acid (HCl), the OH– ion accepts H+ from HCl to produce H2O, which means here LiOH act as a proton acceptor.
So, here, LiOH is not a Bronsted-lowry acid since it is accepting the proton.
Is LiOH alkali or not?
Alkali is a strong base that produces hydroxide ions when it is dissolved in water. All soluble hydroxides like lithium, cesium, sodium, potassium, etc. are alkali metals.
An alkali is said to be strongest when it produces almost all OH- ions when it is dissolved in water.
As for LiOH when dissolving in water it produces almost all OH- ions that ultimately make it strong acid but it is the weakest known alkali metal hydroxide.
Uses of Lithium hydroxide
- It is used in ceramics and some Portland cement.
- It is used as a precursor to making some lithium salts.
- It is used in spacecraft and submarines.
- It is mainly used in the production of cathode materials for lithium-ion batteries.
Summary
- Is LiOH an acid or base? Lithium hydroxide (LiOH) is the base, since, it releases OH– ions on dissolving in an aqueous solution.
- LiOH acting as Arrhenius base and Bronsted-Lowry base.
- LiOH acts as a Lewis base, since, it has an OH– ion that can donate the electron pair to another compound acceptor.
- LiOH is the strong base. Because it completely dissociates in an aqueous solution and produces a large number of OH– ions.
- LiOH is a strong base that forms weak conjugate acid.
- There are three theories to check whether LiOH base or acid. (a). Arrhenius, Bronsted-Lowry, Lewis acid-base theory, etc.
About the author
Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. The team at Topblogtenz includes experts like experienced researchers, professors, and educators, with the goal of making complex subjects like chemistry accessible and understandable for all. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/

