Is Potassium nitrate (KNO3) an acid or base or salt?

Potassium nitrate is also known as an ionic salt of potassium and nitrate ions appear as a white crystalline solid with odorless in nature. It has the chemical formula KNO3. It is easily soluble in water and widely used in making gun powder.
In this article, we will study Is Potassium nitrate (KNO3) is an acid or base or neutral salt?
So, Is KNO3 an acid or base? KNO3 is neither an acid nor base, it is a neutral salt as it is made from the neutralization reaction of the strong acid (HNO3) with a strong base (KOH). The pH value of the aqueous solution of KNO3 is 7. Because strong acid and a strong base will neutralize each other effects and a neutral solution forms.
| Name of Molecule | Potassium nitrate |
| Chemical formula | KNO3 |
| Molar mass | 101.10 g·mol−1 |
| Nature | Neither acid nor base |
| pH | 7 |
Why KNO3 is not acidic in nature?
As we know, the acid compound has a pH value less than 7 and they release H+ ions whenever dissolved in an aqueous solution. In short, an acidic compound is a proton donor which increases the concentration of hydrogen ions in the final solution.
In the case of KNO3, it is not considered an acid because it doesn’t have any H+ or proton ion to donate. So, on dissolving KNO3 in an aqueous solution, it doesn’t induce any effect i.e. Aqueous solution remains the same as before, after dissolving KNO3.
Let’s elaborate more on why KNO3 doesn’t hold the property of acidic with the help of two acid-base theories (a). Arrhenius acid theory (b). Bronsted-Lowry acid theory.
(a). Arrhenius acid theory
According to Arrhenius’s theory of acid ” A compound is said to be acid when added in water, it increases the number of H+ ions in solution.
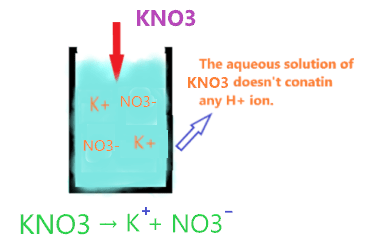
Now, KNO3 on dissolving in aqueous solution, split into two ions K+ and NO3–. As we see, it doesn’t have any H+ ion to donate.
Hence, KNO3 is not qualified as Arrhenius acid as its aqueous solution doesn’t contain any H+ ions.
(b). Bronsted-Lowry acid theory
This theory is an enhanced version of Arrhenius’s theory given for acid-base.
Bronsted-Lowry acid theory state that a compound having the ability to donate the proton to another compound is categorized as Bronsted-Lowry acid.
Since KNO3 doesn’t have any proton to donate. So, we can say KNO3 is neither Arrhenius acid nor Bronsted-Lowry acid as per their acidic theories.
Why KNO3 is not base in nature?
The species having a pH value of more than 7 are said to be basic in nature or “A base is any substance that increases the concentration of OH– ion in aqueous solution”.
Or you can say a base is a compound that accepts hydrogen ions or protons.
So, in the case of KNO3, as like acidic component(H+), it doesn’t have OH– ion also, therefore, dissolving in an aqueous solution does not alter the nature of the solution i.e. it remains the same as before.
As per Bronsted-Lowry base theory, a compound is said to be base when it makes conjugate acid by accepting one proton from other species, or as per Arrhenius base theory, a compound produces OH– ion in an aqueous solution.
“Conjugate acid is formed when one proton is added to parent base”.
Since KNO3 doesn’t have the ability to accept the proton from other species, it cannot make any conjugate acid, nor it produce OH– ions in an aqueous solution.
Therefore, we can say, KNO3 is not a Bronsted-Lowry base as per its theory.
Why KNO3 is neutral salt?
A neutral salt means showing no effect of acidic or alkaline properties when dissolved in water.
Or neutral salt is a salt resulting from the neutralization between acid and base having neither acidic nor alkaline properties when dissolved in an aqueous solution.
A neutralization simply means the interaction of acid and base to form the water molecule and salt compound.

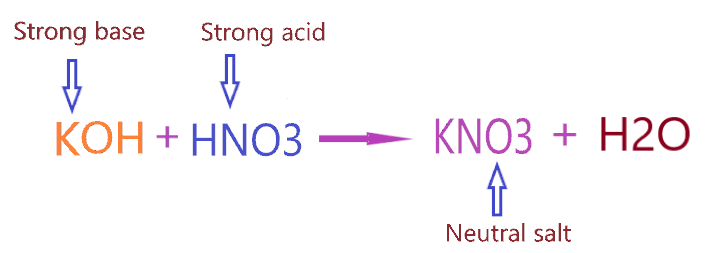
Now let’s come to the point of Why KNO3 is neutral salt? As we know KNO3 salt is formed when the strong base (KOH) and strong acid (HNO3) react with each other. Or you can say KNO3 salt formed by the neutralization reaction carries between HNO3 acid and KOH base.
⇒ KOH + HNO3 → KNO3 + H2O
Also Read:-
As per the concept of the neutralization reaction-
⇒ Strong base + Weak acid = Basic solution
⇒ Stronger acid + Weak base = Acidic solution
⇒ Strong acid + Strong base = Neutral solution
That means the interaction of strong base and weak acid forms a basic solution, stronger acid with weak base forms an acidic solution, and stronger acid with stronger base forms a neutral solution.
As we discussed, KNO3 is formed when strong acid (HNO3) reacts with a strong base (KOH). Hence, as per the above concepts, when a neutralization reaction carries between a strong acid and strong base, it will form neutral salt having a pH value equal to 7.
Using the ionic reaction of potassium nitrate formation we can also understand why the aqueous solution of KNO3 is considered neutral.
We know KNO3 is formed when KOH reacts with HNO3.
⇒ KOH + HNO3 → KNO3 + H₂O
The ionic reaction of the above reaction can be written as-
⇒ K+ + OH– + H+ + NO3– → K+ + NO3– + H2O
Common ions from both(left and right sides) should be canceled out of each other.
Remaining ions we get-
⇒ OH– + H+ → H2O
∴ The final solution of KNO3 contains an equal number of H+ and OH–, therefore, its aqueous solution is neutral.
Also, The ions of KNO3, K+ is the very weak conjugate acid of KOH, and NO3– is a very weak conjugate base of HNO3, hence, both these ions are very weak in nature, therefore, the effect of these ions on altering the pH value of the aqueous solution is almost zero.
The conjugate acid of KOH is K+ which is too weak to function as an acid in water. The conjugate base of HNO3 is NO3– which is too weak to function as a base in water.
This reason can also be considered for explaining why the aqueous solution of potassium nitrate (KNO3) is neutral with no acidic or alkaline properties.
Some examples of neutral salt – NaNO3, NaCl, KCl, KBr, NaBr, etc.
Also Read:
Some examples of acidic salt – NH4NO3, NH4Br, NH4Cl, etc.
Also Read:-
Some examples of basic salt – Na2CO3, NaF, NaCN, Soap, etc.
Also Read:-
The pH value of potassium nitrate
“pH is a measure of hydrogen ion(H+) and hydroxide ion(OH–) concentration in aqueous solution.
Since the water solution of potassium nitrate neither contains H+ ion nor hydroxide ion, so there is no effect on its pH value.
Also, the dissociation of KNO3 into K+ and NO3– ion in water solution have no effect on altering the pH value.
Therefore, the pH value of a water solution of potassium nitrate is 7.
Also Check:
Uses of Potassium nitrate
- It is widely used in making gun powder.
- It is used as a fertilizer and it also works as a strong oxidizing agent.
- It is also used in solid propellants and explosive materials.
- It is used in some toothpaste for sensitive teeth.
- It is used as an electrolyte in salt bridges.
Properties of Potassium nitrate
- It has a melting point of 334 °C and a boiling point of 400 °C.
- It is soluble in water as well as in ethanol, glycerol, and ammonia.
- It has an orthorhombic crystal structure.
- It is generally odorless in nature.
- It is an ionic salt with a pH value equal to near 7.
Summary
Potassium nitrate has no odor appears as a white crystalline solid having a molar mass of 101.10 g·mol−1. It can accelerate the burning of combustible materials. At last, Overview of this article on “Is KNO3 an acid or base or neutral salt?”
- Is KNO3 an acid or base or salt? KNO3 is a neutral salt. It is formed from the neutralization reaction carried out between strong acid, namely Nitric acid (HNO3), and strong base, namely Potassium hydroxide (KOH).
- Potassium nitrate (KNO3) is neither held acidic properties nor alkaline. Because of the absence of the two most important ions needed for acid and base nature, that is H+ and OH–.
- The pH value of potassium nitrate is 7.
About the author
Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. The team at Topblogtenz includes experts like experienced researchers, professors, and educators, with the goal of making complex subjects like chemistry accessible and understandable for all. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/

