Is NH3 (Ammonia) an acid or base? Strong or Weak - Conjugate acid or base pairs

Ammonia appears as colorless gas and has a strong pungent odor having chemical formula NH3. It is made up of one nitrogen and three hydrogen atom. It is one of the important sources of nitrogen for plants and animals. Exposing to open air, liquid ammonia will quickly turn into a gas. Ammonia is easily liquified due to strong hydrogen bonding between molecules.
In this article, we will discuss Is Ammonia (NH3) acid or base? Is it strong or weak? Its conjugate acid-base pairs, etc.
So, Is NH3 an acid or base? NH3 is a weak base. When dissolved in an aqueous solution, it accepts the H+ ion from water molecule ions and produces hydroxide ions (OH–) that correspondingly show its basic nature. However, NH3 can act as acid as well depending on whom it reacts with but primarily, ammonia has a nature of a weak base.
Let’s discuss in detail, in what condition NH3 acts as base and acid, is it lewis acid or base, is it strong or weak, and everything that clears out your all doubt regarding the nature of ammonia.
| Name of Molecule | Ammonia |
| Chemical formula | NH3 |
| pH value | 11 |
| Conjugate acid | NH4+ |
| Conjugate base | NH2– |
| Nature | Weak base |
| Basicity (pKb) | 4.75 |
| Solubility in water | miscible |
Why NH3 is a base?
A base is defined as a proton acceptor or lone pair donor. When NH3 dissolves in water, it will accept the H+ ion from the water ions and gets converted into conjugate acid (NH4+), and produces hydroxide ions (OH–).
The ammonia (NH3) molecule is polar in nature, hence, it is highly soluble in water and able to form a hydrogen bond, therefore, on dissolving NH3 in an aqueous solution, it will fetch the hydrogen ion from H2O and produces hydroxide ions (OH–) and ammonium ions (NH4+)

The production of hydroxide ions on dissolving in an aqueous solution shows the basic nature of ammonia.
Theoretically, we have three acid-base theories to know whether NH3 is acid or base.
(a). Arrhenius theory
(b). Bronsted-Lowry theory
(c). Lewis theory
Let’s check whether NH3 acid or base according to these acid-base theories.
(1). Arrhenius theory:
According to Arrhenius theory, the compound is said to be Arrhenius base when it produces OH– ion through ionization or through dissociation in water and increases the concentration of OH– ions in an aqueous solution. (first definition)
Or a compound is said to be Arrhenius base when the substance contains at least one unit of OH– in the chemical formula and produces OH– ions in water. (second definition)
Clearly, when NH3 is dissolved in an aqueous solution it accepts the proton from the water molecule and produces OH– ion, and from the point of first Arrhenius definition, NH3 will act as Arrhenius base as it is able to increase the concentration of OH– in the final solution.
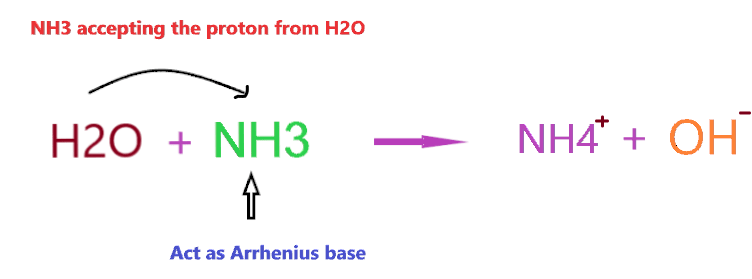
However, if you consider, the 2nd definition of Arrhenius base then NH3 will not act as Arrhenius base because it doesn’t contain any OH– in its chemical formula.
(2). Bronsted-Lowry theory:
According to the Bronsted-Lowry theory, a compound is said to be base when it accepts the proton from other compounds and forms the conjugate acid. Or you can say proton acceptor compounds are classified as Bronsted-Lowry base.
Take an example to understand whether NH3 base or acid according to the Bronsted-lowry theory-
Consider the reaction of NH3 with HCl
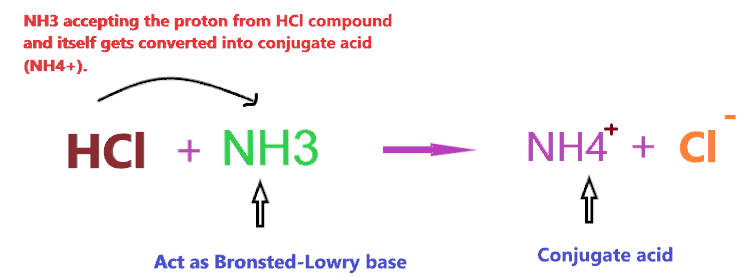
According to the above reaction, when NH3 reacts with a strong acid (HCl), then it will accept the one proton from HCl and itself gets converted into conjugate acid(NH4+), hence, according to the above definition, NH3 will act as Bronsted-Lowry base.
(3). Lewis theory:
Here comes the most important concept of acid and base. According to the lewis theory, a compound is said to be acid when it accepts the pair of electrons and a compound is said to be base when it donates the pair of electrons.
What does it mean?
It means Lewis acid is a compound that has a deficiency of electrons that’s why they are lone pair acceptors and lewis base is a compound that is rich in electrons so, they are lone pair donors.
⇒ Lewis acid → lone pair acceptor
⇒ Lewis base → lone pair donator
The ammonia molecule consists of nitrogen as a central atom that contains two lone pairs of electrons that can be quickly donated to lewis acid because the nitrogen atom is less electronegative, hence, it is always ready to give up the electrons.
Hence, this makes an NH3 molecule serve as a lewis base in nature.
Is NH3 a strong or weak base?
To know whether Ammonia (NH3) is a strong base or weak, you must know the basic difference between a strong base and a weak base.
Strong base: A compound is a strong base when it completely dissociates in an aqueous solution and liberates a large number of hydroxide ions. All moles of the strong base dissociates into hydroxide ion(OH–) and no part remains undissociated in the solution.
Example: Sodium hydroxide(NaOH), Barium hydroxide (Ba(OH)2), Calcium hydroxide (Ca(OH)2), Lithium hydroxide (LiOH), Potassium hydroxide (KOH), etc.
Also, Read:–
- Why KOH is a strong base?
- Why Ba(OH)2 is a strong base?
- Why Ca(OH)2 is a strong base?
- Why NaOH is a strong base?
Weak base: A compound is a weak base when it partially or not completely dissociates in an aqueous solution which means not all moles of the base dissociate in a solution to yield OH– ion, and at equilibrium, both undissociated base and their ionized product present in the solution.
Example- Ammonia hydroxide (NH4OH), Methylamine ( CH3NH2), etc.
Also, Read:–
| Strong base | Weak base |
| They ionize completely. | They do not completely ionize. |
| They are highly reactive. | They are less reactive compared to a strong base. |
| The value of pH lies between 10 to 14. | The value of pH lies between 7 to 10. |
| They have a high equilibrium constant. | They have a less equilibrium constant. |
| They are good electrolytes. | They are not so good electrolytes compared to a strong base. |
| Example – NaOH, KOH, LiOH, etc. | Example – N2H4, NH4OH, etc. |
So, Is Ammonia (NH3) a strong base or weak? NH3 is considered a weak base because when it is dissolved in an aqueous solution then not all the molecules of it react with water to yield OH– ions, very few molecules of NH3 react with water molecule ions and produce OH– ions in the solution.
Therefore, the amount of OH– ions produced in an aqueous solution is very low as compared to the number of NH3 moles we dissolved in the solution.
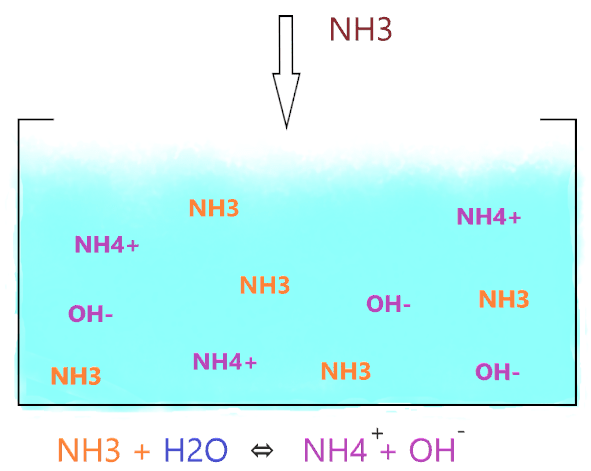
As shown in the figure, when NH3 is dissolved in water, it accepts the hydrogen ion from the water molecule and produces two ions(NH4+ and OH–) but the ion(NH4+) is not stable in an alkaline environment, it keeps breaking into NH3 and H+.
⇒ NH4+ ⇔ NH3 + H+
The H+ ions react with OH– and forms H2O.
⇒ NH3 + H2O ⇔ NH4+ + OH–
The double arrow in the reaction of ammonia and water shows that both forward and backward reactions occur at equilibrium.
But the the favor of reaction mostly lies to the left side that means large number of NH3 will present in the aqueous solution as compare to its right sided product(NH4+ and OH–)
Hence, not all the NH3 molecules react with water ions and produce OH– ions, most of them just stay as NH3, only, a few molecules do interact with water, therefore, ammonia is considered a weak base in nature.
More reasons, why is NH3 is considered a weak base? Ammonia has a very small value of base dissociation constant(Kb).
“The base dissociation constant value is used to distinguish the strong base from the weak base”. The higher the value of Kb, the more the base dissociates.
Also, ammonia is a stable compound in its original form(NH3) and it doesn’t like to accept the proton to form NH4+. So, NH3 is behaving as a weak base as it doesn’t readily accept the proton ion in an aqueous solution.
Let’s understand why NH3 acts as the weak base with the help of the dissociation constant value concept.
⇒ If the value of the dissociation constant of acid is greater than 1 (Ka > 1), then the nature of the compound is a strong acid.
⇒ If Ka < 1, then the nature of the compound is a weak acid.
⇒ If Kb >1, then the nature of the compound is a strong base.
⇒ If Kb <1, then the nature of the compound is a weak base.
To figure out the dissociation constant value of NH3, first, we should know the Ka value for NH4+.
∴ The dissociation constant value(Ka) for NH4+ is 5.56 × 10-10.
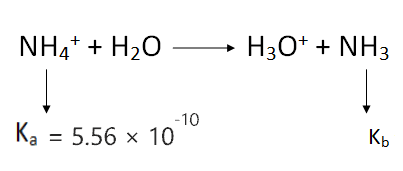
∴ Kb = Kw/Ka
⇒ Kb = 10-14/ 5.56 × 10-10 [∴ Kw = 10-14]
∴ Kb for ammonia = 1.8 × 10-5
The value of Kb for NH3 is way lower than 1, therefore NH3 is a weak base in nature.
Here’s the list of some common acids and bases with their strength.

Also check:-
Is NH3 also act as acid?
So, Is NH3 also act as an acid? Ammonia (NH3) can act as acid only in one condition when the reacting compound is more basic than it e.g. OH–. This is because a stronger base dissociates in an aqueous solution and strongly accepts the proton.
Therefore, when NH3 reacts with a stronger base like OH–, it is left with no choice and has to release a proton. And we know anything that releases the proton is classified into the acid category.
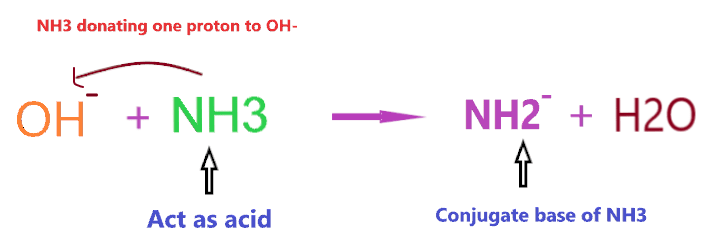
As we see in the above reaction, NH3 reacts with a strong base(OH–), therefore liberating one proton to hydroxide ion and making an amide anion(NH2–).
According to the Bronsted-Lowry theory for acid said that “the acid is the substance that donates the proton to reacting species and itself makes a conjugate base.”
So, in the case of NH3, it donates the proton to reacting species(OH–) and also makes a conjugate base(NH2–).
Therefore, we can say NH3 (ammonia) can act as Bronsted-Lowry acid when reacts with strong bases.
Why NH3 act as a Lewis base?
Lewis’s theory is a very important acid-base theory to check whether a compound (NH3) is acid or base?
According to the Lewis theory, a compound is said to be acid when it accepts the pair of electrons and a compound is said to be base when it donates the pair of electrons.
⇒ Lewis acid → lone pair acceptor
⇒ Lewis base → lone pair donator
Now, why does NH3 acts as a Lewis base? To know this first we should know what type of compound is Lewis acid and Lewis base.
Lewis acid: They are electron-loving compounds and are also known as electrophilic compounds that accept the electron pair from Lewis bases.
Some examples of Lewis acids that can accept the pair of electrons from another species.
- The molecules or ions with an incomplete octet of electrons. Example – BF3, BCl3, AlF3, etc.
- The molecule that central atom has an empty d-orbital. Example- SiCl4 (1s22s22p63s23p2 3d0).
- The compounds that the central atom formed multiple bonds with the adjacent atoms. Example – SiO2 (O = Si = O), CO2, SO2, etc.
- Simple cations like H+, Na+, Mg+2, Al+3, etc. are lewis acids since they are able to accept the electrons. ( Exception NH4+, PH4+ are not a lewis acid)
Lewis base: They are electron-rich species and also known as nucleophile compounds that donate the electron pairs to the Lewis acids.
Some examples of Lewis bases that can donate the pair of electrons to other species.
- The negative ion such as H–, OH–, F–, etc.
- Species that have lone pair of electrons. Example – NH3, H2O, CH3–, etc.
Now ammonia (NH3) acts as lewis’s base because the central nitrogen atom has two lone pairs of electrons and it is always ready to give up the lone pair of electrons to another compound as it is very less electronegative in nature.
Let’s understand it with the help of an example-

The electrons in the hydrogen-fluorine bonds attracted towards the fluoride ion because of it high electronegativity leaving hydrogen slightly positive and fluorine negative.
The lone pair on the nitrogen of NH3 molecule attracted towards hydrogen atom in HF molecule.
As it approaches it, the electrons in the hydrogen-fluorine bond are repelled still further towards the fluorine.
Eventually, a co-ordinate bond is formed between the nitrogen and the hydrogen, and the fluorine breaks away as a fluoride ion.
The whole HF molecule acts as Lewis acid as it accept the lone pair from nitrogen atom, and in this process it breaks up. [Lewis acid & base guide check here)
So, HF accepts the lone pair of the electron, therefore, it is Lewis acid and NH3 donates the lone pair of the electron, therefore, it is Lewis base.
“Ammonia, NH3, is a Lewis base and has a lone pair. It will donate electrons to compounds that will accept them. Donation of ammonia to an electron acceptor, or Lewis acid. There may be anionic or neutral Lewis bases.”
What is the conjugate acid of NH3?
In technical terms, Compounds differentiated from each other by a single proton(H+) are said to be Conjugate acid-base pairs.
In simple terms, when the proton is removed from parent acid then the compound is formed which is called the conjugate base of that acid and when the proton is added to the parent base then the compound is formed which is called conjugate acid of that base.
Concept of Conjugate acid-base pair-
- A very weak acid forms the strong conjugate base.
- A very weak base forms strong conjugate acid.
- A very strong acid forms the weak conjugate base.
- A very strong base forms weak conjugate acid.
⇒ Very weak means it doesn’t act as acid or base when dissolved in an aqueous solution.
⇒ Very strong means, acid or base ionizes 100% when dissolved in an aqueous solution.
Example 1: HCl → H+ + Cl–
In this reaction, HCl is a very strong acid and we know very strong acid always forms the weak conjugate base by donating one proton.
So, Cl– is the weak conjugate base of HCl.
Example 2: CH3COOH + H2O → CH3COO– + H3O+
Here in this reaction, CH3COOH is a weak acid that donates the proton to form CH3COO–, which means CH3COO– is the conjugate base of CH3COOH.
H2O behaves as the base because it accepts the proton from CH3COOH to form H3O+, which means H3O+ is the conjugate acid of H2O.
So, what is the conjugate acid of NH3? As we discussed earlier, NH3 is a weak base, hence, it will form a conjugate acid by adding one proton to itself.
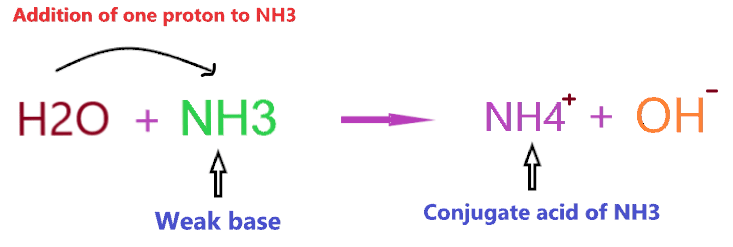
As you see in the above reaction, NH3 is a weak base and we know a weak base always forms a conjugate acid(not necessarily the strong one).
How to know if NH3 is acid or base practically?
Till now we learn how to know if compound acid or not theoretically. To know practically, one of the easiest ways to use litmus paper.
“Litmus is a water-soluble mixture of different dyes extracted from lichens. It is often absorbed onto filter paper to produce one of the oldest forms of pH indicator, used to test materials for acidity.”
Note: When Red litmus paper turns blue then the compound is said to be base. And when blue litmus paper turns red then the compound is said to be acidic.
When NH3 is contacted with red litmus paper then litmus paper turns into blue color. So, we can say NH3 is the base.
FAQ
Which is more stable NH4+ or NH3? |
Always remember (A neutral molecule is more stable than any charged molecule). So, we can say from NH4+ and NH3, NH3 is more stable as it doesn’t have any charge on it. But the stability of a molecule depends on the medium i.e. Alkaline or Acidic. In an acidic environment, NH4+ is more stable than NH3 and in an alkaline environment, NH3 is more stable than NH4+ because NH3 is a weak base in nature and NH4+ is a weak acid. If there is no medium mentioned then an uncharged molecule is always more stable than a charged molecule. |
Why does NH3 behave as amphoteric species? |
Amphoteric species are the species or compounds that act as acid as well as a base depending on the condition. Ammonia(NH3) has an amphoteric nature as it acts as a weak base when reacts with acidic compounds and forms conjugate acid(NH4+) by accepting the proton. Also, ammonia acts as a weak acid when reacts with a stronger base like OH– and forms a conjugate base(NH2–) by donating the proton. ⇒ NH3 + HF → NH4+ + F– (∴NH3 acting as base) ⇒ NH3 + OH– → NH2– + H2O (∴ NH3 acting as acid) |
Conclusion
I hope you must enjoy this article on Is Ammonia (NH3) is an acid or base? And I think this article clears all of the doubt you have over Why NH3 is base, why it’s also acts like acid, is a strong or weak base, etc. Still, if you have a doubt regarding this then you must clear it by asking in a comment box. Lastly, I am giving you a short overview of this article.
- Is ammonia (NH3) an acid or base? Ammonia is primarily considered a base. Because the nitrogen central atom consists of one lone pair which can be used to accept the proton, hence, this leads to the formation of NH4+ and OH– when ammonia is dissolved in water. The presence of OH– in the aqueous solution results in a pH increase and this makes the aqueous solution of ammonia (NH3) basic in nature.
- Ammonia (NH3) is considered as Arrhenius base, Bronsted-Lowry base, and Lewis base, due to having the ability “to increase the concentration of OH– ions in water”, “accept a proton from another compound”, having the ability to “donate the lone pair of electrons”.
- When NH3 acts as a base then it forms conjugate acid(NH4+) and when it acts as acid then it forms the conjugate base(NH2–).
- NH3 can also act as an acid when reacting with a stronger base than it and forms a conjugate base(NH2–) by releasing one proton. This shows the nature of NH3 towards amphoteric species.
- Ammonia (NH3) is considered a weak base because not all the NH3 molecules react with water ions and produce OH– ions, most of them stay together, only, a few molecules do interact with water, Therefore, the amount of OH– ions produced in an aqueous solution is very low as compared to the number of NH3 moles we dissolved in the solution.
- “In ammonia, the nitrogen atom has a lone pair of electrons that can be quickly donated to the necessary Lewis acid. Ammonia will thus serve as a Lewis base.”
- The base dissociation constant value for NH3 is approximately 1.8 × 10-5 which is way less than 1 and that shows its weak base nature.
About the author
Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. The team at Topblogtenz includes experts like experienced researchers, professors, and educators, with the goal of making complex subjects like chemistry accessible and understandable for all. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/