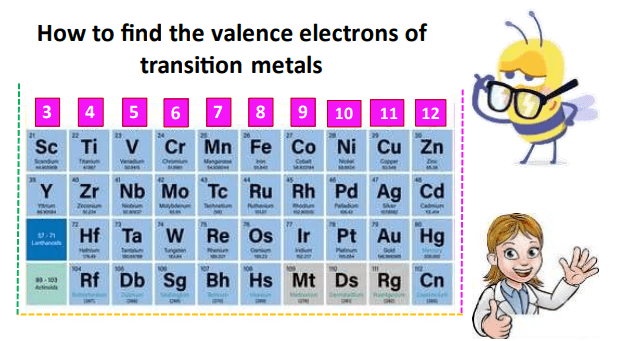
How to find Valence electrons of transition metals (d-block elements)?
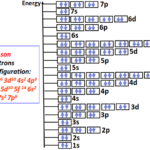
Transition metals lie at the center of the Periodic Table of elements in the d-block. Transition metals possess several interesting properties such as they can form ions with different charges. Some characteristic transition metal ions in turn can form brightly colored solutions.
The electronic structure and the number of valence electrons found in a transition metal element play a central role in controlling all of its fascinating properties. However, a chemistry student often stays confused about how to find the valence electrons of transition metals.
But don’t worry because this article will clear all such doubts. So, let’s begin reading.
How to find valence electrons of transition metals?
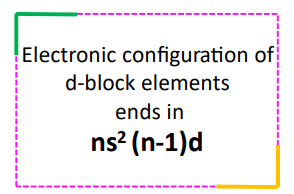
The valence electrons of a transition metal element can be determined from its ground state electronic configuration. The electronic configuration of transition metals is predicted by the building-up principle. It usually ends in ns2 (n-1)d. So, the total valence electrons present in the transition metal are the sum of the electrons present in its ns and (n-1) d orbitals.
The total valence electron value often coincides with the group number of the element. For example, iron (Fe) has a total of 8 valence electrons, and it is present in Group 8 of the modern Periodic Table.
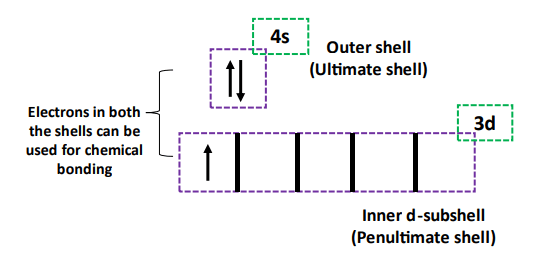
Generally, valence electrons are referred to as the electrons present in the outermost shell of an elemental atom. In the case of main group elements (s and p block), this definition is sufficient because usually, the electrons present in the outermost shell are also the ones available for chemical bonding.
But in the case of transition metals, this definition lags behind. This is because, in addition to the electrons present in the outermost shell, the transition metal elements can involve in chemical bonding, and their inner shell electrons as well.
How to write the electronic configuration of transition metals?
The d-block transition metals lie in groups 3-12 of the Periodic Table. According to the building-up principle, after calcium (Z=20), the electrons start to occupy the 3d subshell. The 3d subshell is considered an inner shell relative to 4s therefore the name d-block is given to transition metal elements.
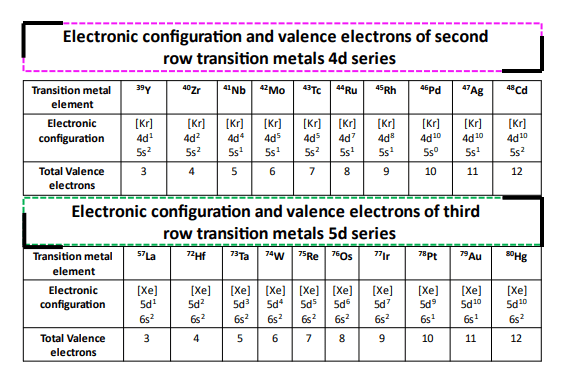
The first-row transition metals (Period 4) all have their valence electrons placed in 3d and 4s subshells. For the subsequent rows (Period 5-7), the valence electrons occupy 4d, 5d, and 6d inner shell (n-1) orbitals respectively while having 5s, 6s, and 7s as their outermost (n) shells.
Electrons occupy transition metal orbitals following the same three rules that are assigned for the main group elements.
- Aufbau principle: In all cases, the electrons first occupy the ns shell and then fill into the (n-1)d subshell
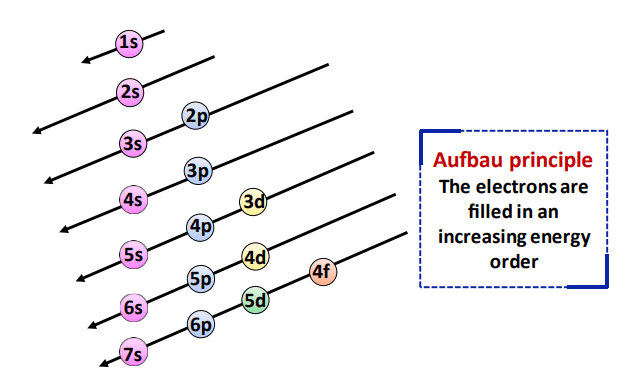
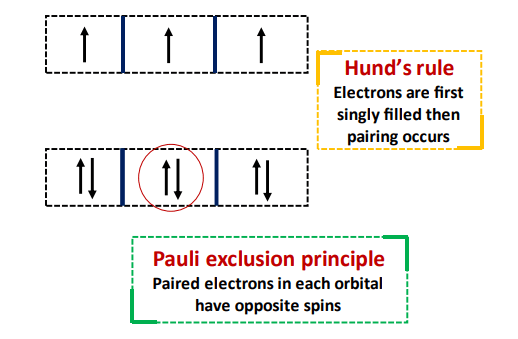
- Hund’s Rule: The electrons are first singly filled and then pairing occurs.
- Pauli Exclusion principle: The ns subshell can hold a maximum of 2 electrons in opposite spins. Conversely, the (n-1)d subshell can accommodate a maximum of 10 electrons as 5 pairs, one in each orbital.
 Electronic configuration of first-row transition metals
Electronic configuration of first-row transition metals
The first-row transition metals (Sc to Zn) are extremely important based on their applications in industry and everyday life. Therefore, we would like to introduce you to their electronic configuration so that you can easily find how many electrons are present in each.
Steps to write the electronic configuration of Scandium (21Sc)
Step I: Identify the total electrons present in the Scandium atom in the ground state.
Its atomic number is Z=21 so it has 21 electrons present in it in the ground state.
Step II: Write the electronic configuration using the three rules discussed above, until you reach the 3p orbital.
21Sc = 1s2 2s2 2p6 3s2 3p6
Step III: Identify the electrons still left after filling the 3p orbital.
Electrons already filled: 2+2+6+ 2+6 = 18
Electrons still left: 21-18 = 3
Step IV: Two of the remaining electrons will occupy the lower energy 4s orbital while the rest will be placed in the 3d orbital.
2 electrons filled in 4s of Sc leaves behind 3-2 = 1 electron only. This single electron will be situated in the 3d orbital.
So, the electronic configuration of Sc is: 1s2 2s2 2p6 3s2 3p6 3d1 4s2
Step V: Identify the valence electrons as the sum of electrons present in ns (in this case 4s) and (n-1)d (in this case 3d) orbitals.
1+2 = 3 valence electrons are present in Scandium.
You can apply the same steps for other transition metal elements to find their valence electrons and check your results against the table given below.
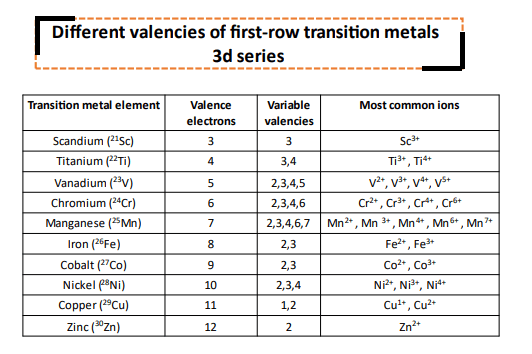
Valence electrons of transition elements |
|
| Transition metals (d block elements) | Total Valence electrons |
| Scandium (Sc) | 3 |
| Titanium (Ti) | 4 |
| Vanadium (V) | 5 |
| Chromium (Cr) | 6 |
| Manganese (Mn) | 7 |
| Iron (Fe) | 8 |
| Cobalt (Co) | 9 |
| Nickel (Ni) | 10 |
| Copper (Cu) | 11 |
| Zinc (Zn) | 12 |
Note: [Ar] represents the electronic configuration of Argon (18Ar) the Noble gas
i.e., 1s2 2s2 2p6 3s2 3p6
Exceptions
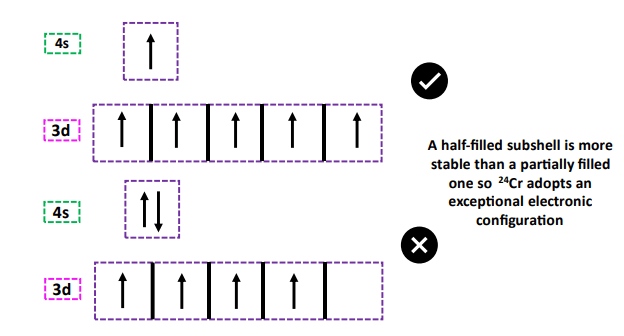
From the table above, you must have noticed that in the electronic configuration of chromium (Cr) and copper (Cu), there is only 1 electron placed in the 4s (outer shell) orbital after which filling starts in the (3d) inner subshell.
The transition metal elements in groups 6 and 11 are thus called exceptions.
This is because a half-filled 3d5 or a completely filled 3d10 subshell is more stable than the partially filled d4 or d9 respectively. However, it does not effect the total valence electrons present in each case.
Variable valencies of transition metal elements
We discussed at the beginning of this article that transition metal elements can involve their inner shell valence electrons in addition to the outer electrons in chemical bonding.
Therefore, transition metal elements exhibit variable valencies. They can form more than one type of ion.
For example, Iron (Fe) has a total of 8 valence electrons that it can use for chemical bonding. It most commonly loses its two 4s electrons from the outermost shell to form Fe2+ (ferrous) ions. But, it can also lose three valence electrons (two electrons from 4s and 1 electron from the inner 3d orbital to from Fe3+ (ferric) ions.

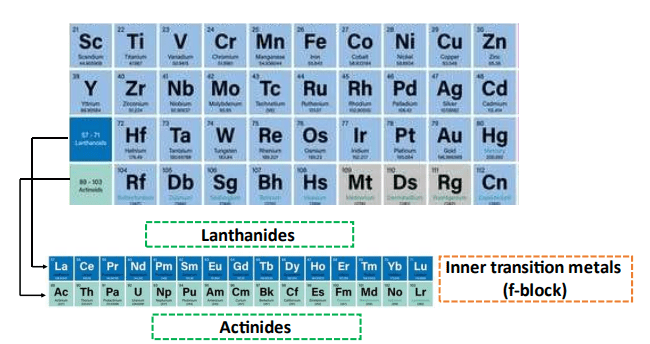
Valence electrons present in inner transition metal elements (f-block)
Lanthanides and actinides separately situated at the bottom of the Periodic Table in the f-block are known as inner transition metals. This is because they have atomic numbers that lie between the atomic numbers of the first and second elements in the last two rows of the d-block.
The electronic configuration of Lanthanides ends in 4f1-14 5s2 5p6 5d0-1 6s2 while that of Actinides ends in 5f1-14 6s2 6p6 6d0-1 7s2. Electrons occupy f sub-shells therefore the name f-block is given.
However, electrons occupy shells and sub-shells depending upon the energy differences. Electrons are filled first in orbitals lying low in energy followed by high-energy orbitals (Aufbau principle).
Thus, you will find unexpected electronic configurations in this block. But once their electronic configuration is known, based on that, we can find the valence electrons of inner transition metals.
Examples
| Inner transition metal | Cerium (58Ce) | Thorium (90Th) |
| Electronic configuration | [Xe] 4f1 5d1 6s2 | [Rn] 6d27s2 |
| Valence electrons | 1+1+2=4 | 2+2 =4 |
Also check:
FAQ
How do you find the valence electrons of d-block elements? |
| The valence electrons of d-block elements can be determined from their electronic configuration. These are the electrons that the element can involve in chemical reactions. Generally, it is the sum of electrons present in outer ns and inner (n-1)d orbitals of the d-block elements. |
How to find the valence electrons of first-row transition metals? |
| The valence electrons of first-row transition metals lie in 4s and 3d orbitals. For example, the electronic configuration of Vanadium (23V) is [Ar] 3d3 4s2 so it has a total of 3+2 =5 valence electrons. 5 is also its group number. |
Why do we consider (n-1) d electrons as valence electrons in transition metals? |
| The transition metals can involve their penultimate shell (n-1)d electrons in chemical bonding in addition to the ns electrons. Therefore, (n-1)d electrons are also considered valence electrons. |
Why don’t transition metals use all their valence electrons in bonding? |
|
Transition metals form positively charged ions by the loss of their valence electrons, prior to chemical bonding. It is due to the force of attraction between the positively charged nucleus and the negative electrons that the transition metal atom cannot lose all of its valence electrons at once for bonding. |
What is the difference between the valence electrons and the valency of transition metals? |
|
The valence electrons are the total electrons available for chemical bonding while valency refers to the combining power of the transition metal element with other elements. The transition metal may not always use all of its electrons for bonding so its valency can be different from the valence electrons present in it. The valence electrons of a transition metal is a specific integer value, contrarily it can exhibit more than 1 valency. |
Why do all d-block elements theoretically have 2 valence electrons? |
|
The electronic configuration of all d-block elements (except Cr and Cu) ends in ns2. This means there are two valence electrons in the outermost (ultimate) shell, therefore, in line with the conventional definition of a valence electron, all d-block elements have 2 valence electrons. It should also be noted that – “Most transitional metals have two valence electrons, but may have a larger range of apparent valence electrons. Locate the transition metal on the periodic table and make note of the group number.” |
Summary
- Transition metals are situated in the d and f blocks of the Periodic Table.
- In addition to their outer-shell electrons, the transition metal elements can involve their inner-shell electrons in chemical bonding.
- The inner d-subshell electrons of transition metals are also called valence electrons.
- So, the valence electrons of a transition metal can be determined using its electronic configuration, by taking the sum of its ns and (n-1)d electrons.
- First-row transition metals have a similar electronic configuration that ends in 3d1-10 4s2.
- 24Cr and 29Cu are exceptions with a half-filled 4s1 atomic orbital.
About the author
Ammara Waheed is a highly qualified and experienced chemist, whose passion for Chemistry is evident in her writing. With a Bachelor of Science (Hons.) and Master of Philosophy (M. Phil) in Physical and Analytical Chemistry from Government College University (GCU) Lahore, Pakistan, with a hands-on laboratory experience in the Pakistan Council of Scientific and Industrial Research (PCSIR), Ammara has a solid educational foundation in her field. She comes from a distinguished research background and she documents her research endeavors for reputable journals such as Wiley and Elsevier. Her deep knowledge and expertise in the field of Chemistry make her a trusted and reliable authority in her profession. Let's connect - https://www.researchgate.net/profile/Ammara-Waheed