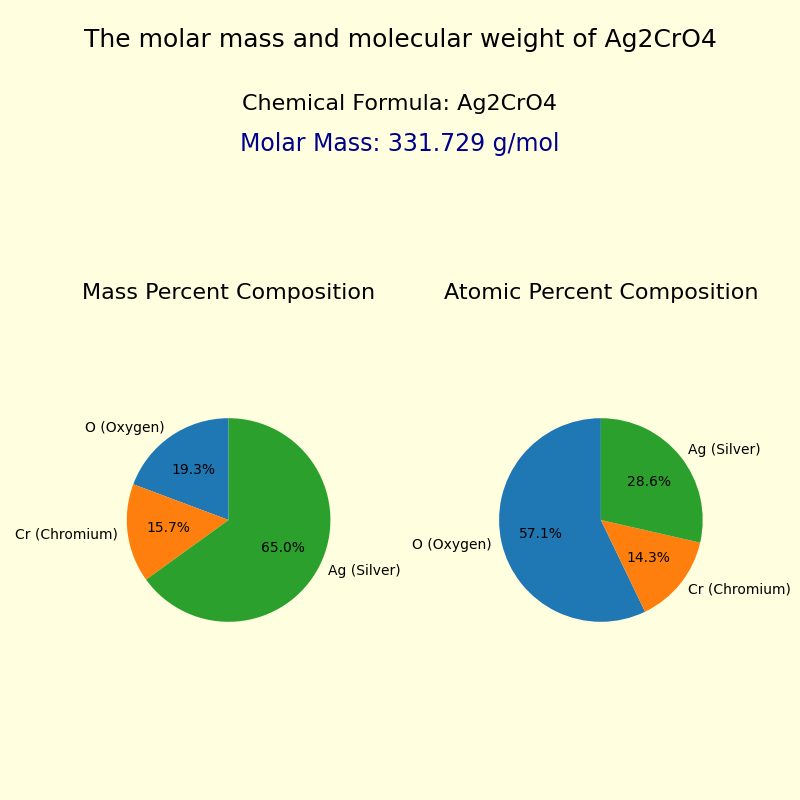
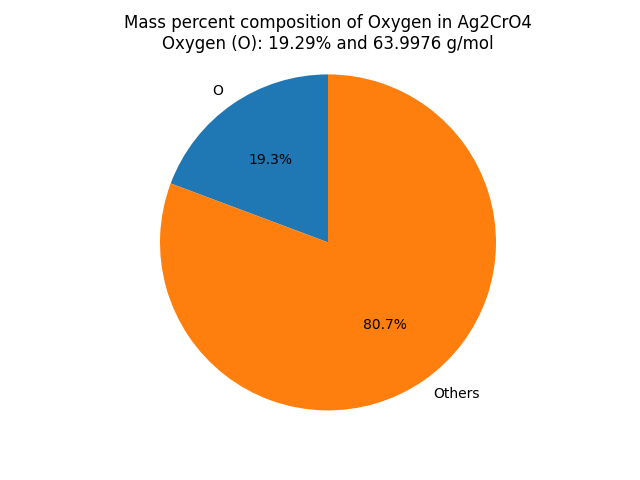The molar mass and molecular weight of Silver Chromate (Ag2CrO4) is 331.7296 g/mol.
The composition of the Ag2CrO4 formula is as follows:
| Element | Symbol | Atomic Weight | Atoms | Total Atomic Weight | Mass Percent |
|---|---|---|---|---|---|
| Oxygen | O | 15.9994 g/mol | 4 | 63.9976 g/mol | 19.2921% |
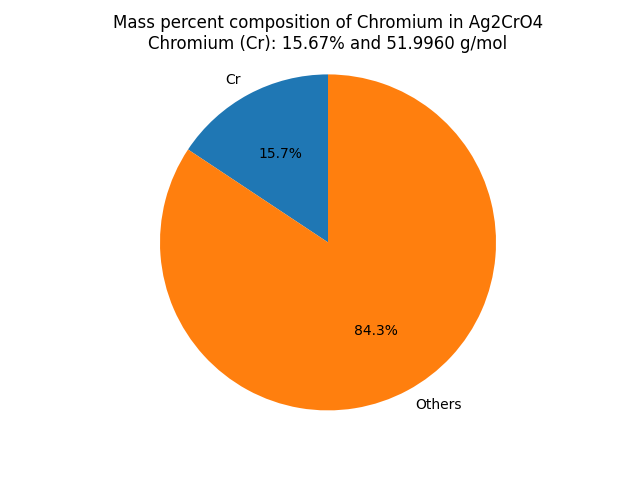
| Chromium | Cr | 51.996 g/mol | 1 | 51.9960 g/mol | 15.6742% |
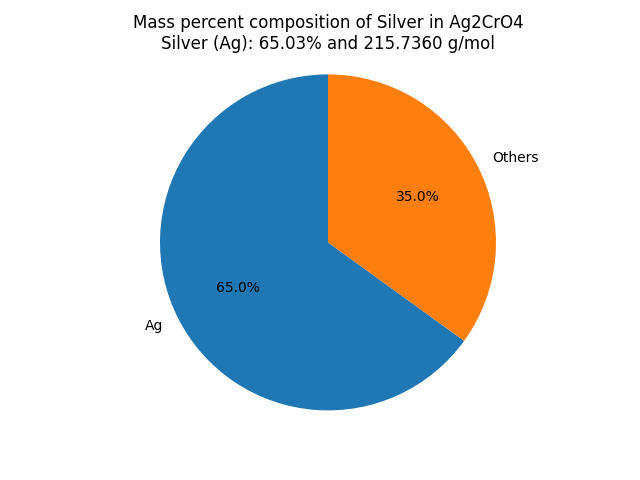
| Silver | Ag | 107.868 g/mol | 2 | 215.7360 g/mol | 65.0337% |
How to find the molar mass of Silver Chromate (Ag2CrO4)?
The molar mass and molecular weight of Silver Chromate (Ag2CrO4) can be calculated in 4 steps.
Step I: Identify the different elemental atoms present in the Ag2CrO4 compound.
The given compound is Ag2CrO4. It comprises atoms from 3 different elements i.e., Oxygen (O), Chromium (Cr) and Silver (Ag).
Step II: Find the atomic weight of each element in the Ag2CrO4.
Here is the list of atomic weights for all elements, let’s check the Oxygen (O), Chromium (Cr) and Silver (Ag) atom’s atomic weight.
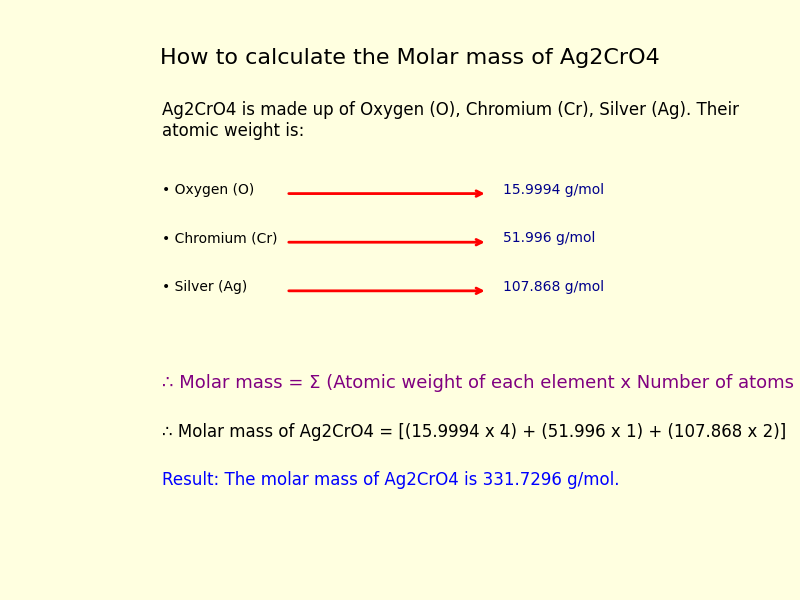
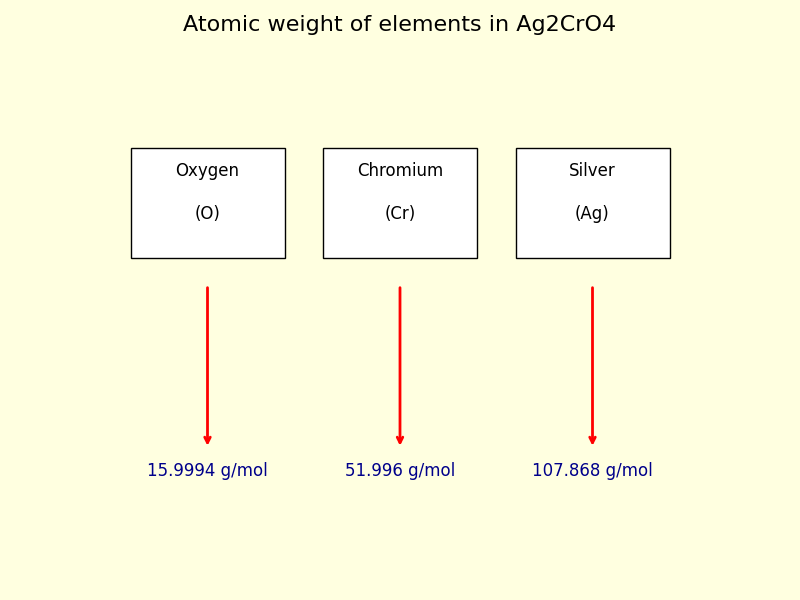
• The atomic weight of Oxygen (O) is 15.9994 g/mol.
• The atomic weight of Chromium (Cr) is 51.996 g/mol.
• The atomic weight of Silver (Ag) is 107.868 g/mol.
Step III: Determine the number of atoms of each element present in the Ag2CrO4 compound.
As per the chemical formula, Ag2CrO4, It is made up of 4 Oxygen atoms, 1 Chromium atom and 2 Silver atoms.
| Element | Number of Atoms |
|---|---|
| O (Oxygen) | 4 |
| Cr (Chromium) | 1 |
| Ag (Silver) | 2 |
Step IV: Calculate the molar mass of the Silver Chromate (Ag2CrO4) compound by applying the formula:

For Silver Chromate (Ag2CrO4):
Substituting into the above formula, the values determined in steps II and III:
Molar mass of Ag2CrO4 = [(15.9994 x 4) + (51.996 x 1) + (107.868 x 2)]
Molar mass of Ag2CrO4 = 63.9976 + 51.996 + 215.736 = 331.7296 g/mol
Result: The molecular weight and molar mass of Silver Chromate (Ag2CrO4) is 331.7296 g/mol.
FAQs
What is the mass percent composition of Oxygen (O) in Silver Chromate (Ag2CrO4)? |
To find the mass percent of Oxygen in Ag2CrO4, follow the steps given below:
∴ Mass of Oxygen in Ag2CrO4 = 15.9994 x 4 = 63.9976 g/mol. Mass Percent Composition (%) Formula = (Mass of Element in the Compound/Molar Mass of the Compound) x 100 ∴ Mass percent of Oxygen in Ag2CrO4 = (Mass of Oxygen in Ag2CrO4/Molar mass of Ag2CrO4) × 100% Result: Ag2CrO4 contains 19.29 % of Oxygen as per its chemical composition.
|
What is the mass percent composition of Chromium (Cr) in Silver Chromate (Ag2CrO4)? |
To find the mass percent of Chromium in Ag2CrO4, follow the steps given below:
∴ Mass of Chromium in Ag2CrO4 = 51.996 x 1 = 51.9960 g/mol. Mass Percent Composition (%) Formula = (Mass of Element in the Compound/Molar Mass of the Compound) x 100 ∴ Mass percent of Chromium in Ag2CrO4 = (Mass of Chromium in Ag2CrO4/Molar mass of Ag2CrO4) × 100% Result: Ag2CrO4 contains 15.67 % of Chromium as per its chemical composition.
|
What is the mass percent composition of Silver (Ag) in Silver Chromate (Ag2CrO4)? |
To find the mass percent of Silver in Ag2CrO4, follow the steps given below:
∴ Mass of Silver in Ag2CrO4 = 107.868 x 2 = 215.7360 g/mol. Mass Percent Composition (%) Formula = (Mass of Element in the Compound/Molar Mass of the Compound) x 100 ∴ Mass percent of Silver in Ag2CrO4 = (Mass of Silver in Ag2CrO4/Molar mass of Ag2CrO4) × 100% Result: Ag2CrO4 contains 65.03 % of Silver as per its chemical composition.
|
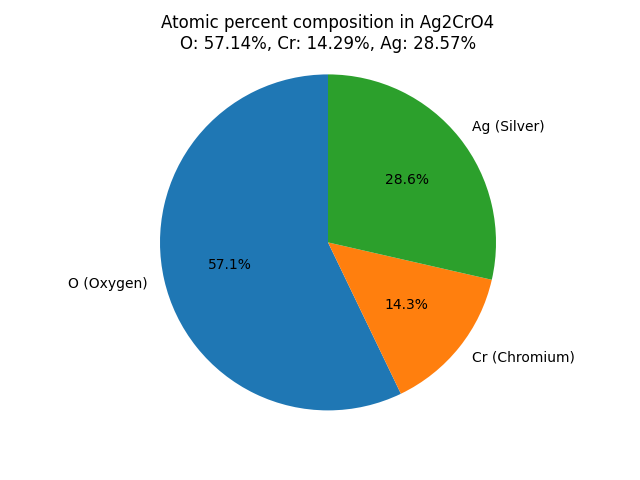
What is the atomic percentage composition of elements in Silver Chromate (Ag2CrO4)? |
| Atomic percentage composition formula (%) = Number of atoms of a particular element in a compound/Total number of atoms in that compound
For Ag2CrO4: The atomic percentage composition of elements in the compound is: Atomic percentage of Oxygen (O) in Ag2CrO4: Atomic percentage of Chromium (Cr) in Ag2CrO4: Atomic percentage of Silver (Ag) in Ag2CrO4:
|
How many grams of Oxygen are present in 1 mole of Ag2CrO4? |
| There are 4 O-atoms in a Ag2CrO4 molecule. • Atomic weight of a O-atom = 15.9994 g/mol ∴ Total mass of Oxygen in 1 mole of Ag2CrO4 = 4 x 15.9994 = 64.00 g. Therefore there are 64.00 grams of Oxygen in 1 mole of Ag2CrO4. |
What is the mass of 7 mol of Ag2CrO4? |
| The molecular mass of Ag2CrO4 is 331.7296 g/mol. This means 331.7296 grams of Ag2CrO4 are present per mole. Therefore, we can find the mass of Ag2CrO4 in 7 moles as follows: ∴ Moles = Mass/Molar Mass ∴ Mass of Ag2CrO4 = Moles x Molar mass ∴ Mass of Ag2CrO4 = 7 x 331.7296 = 2322.11 g Thus, the mass of 7 mol of Ag2CrO4 is 2322.11 g. |
If you have 82.3453 grams of Ag2CrO4, how many moles do you have? |
| ∴ Moles = Mass/Molar mass ∴ Moles of Ag2CrO4 = 82.3453/331.7296 = 0.2482 Thus, there are 0.2482 moles of Ag2CrO4 in its 82.3453 grams. |
What is the mass in kg of 55.29 x 1025 molecules of Ag2CrO4? |
| 1 mole of a substance contains Avogadro number of particles i.e., 6.02 x 1023. Therefore, the number of moles in 55.29 x 1025 molecules of Ag2CrO4 are: ∴ Moles of Ag2CrO4 = 55.29 x 1025/6.02 x 1023 = 918.4
Now that we have its number of moles, so, we can use the molar mass of Ag2CrO4 (331.7296 g/mol) to find its mass as shown below. ∴ Mass of Ag2CrO4 = Moles x Molar mass
∴ Mass of Ag2CrO4 = 918.4 x 331.7296 = 304663.70 g
Converting mass from grams (g) to kilograms (kg) gives us: ∴ Mass of Ag2CrO4 = 304663.70/1000 = 304.66 kg
The mass of 55.29 x 1025 molecules of Ag2CrO4 is 304.66 kg.
|
What is the molar mass and molecular weight of Silver Chromate (Ag2CrO4)? |
Ag2CrO4 is composed of 4 Oxygen (O), 1 Chromium (Cr), and 2 Silver (Ag) atoms.
To find the molecular mass of Ag2CrO4, one multiplies the atomic weight of each element by its number of atoms in the molecule and then sums the results. ∴ For Ag2CrO4, it’s (4 x 15.9994) + (1 x 51.9960) + (2 x 107.8680).
Therefore, the molar mass of Silver Chromate (Ag2CrO4) is 331.7296 g/mol.
|
About the author
Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. The team at Topblogtenz includes experts like experienced researchers, professors, and educators, with the goal of making complex subjects like chemistry accessible and understandable for all. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/