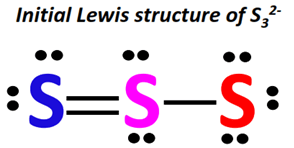The question is –
How many Lewis resonance structures are possible for S32-?
Answer:
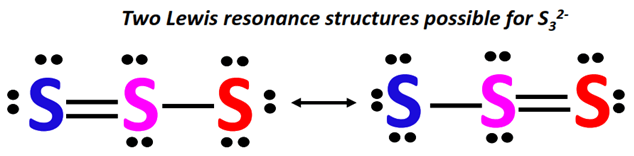
⇒ Two equivalent Lewis resonance structures can be drawn for S32- as shown below.
Explanation:
S32- represents the trisulfide anion, commonly known as polyatomic trisulfide. It consists of three sulfur (S) atoms.
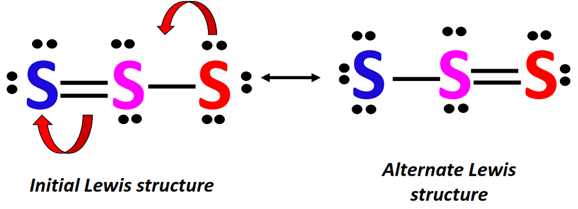
The three S-atoms are arranged linearly in the Lewis dot structure of S32-, which has a total of 20 valence electrons.
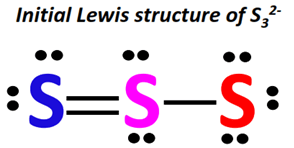
AnS-atom at the center is double-covalently bonded to another S-atom on one side and single-bonded to the third S-atom on the other side.
2 lone pairs of electrons are present on each of the central S-atom and the double-bonded terminal S-atom.
In contrast, there are 3 lone pairs on the single-bonded S-atom in an S32- Lewis structure.
The terminal S-atoms act as resonance contributing atoms.
A lone pair from the single-bonded S-atom (marked red) flows to the central S-atom to form an extra bond between the respective S-atoms.
This increase in electron density on one side of the ion is balanced by a simultaneous decrease in electron density on the other side as the already present S=S bond breaks into single.
An extra lone pair is placed on the S-atom (marked blue) whose double bond is broken.
In this way, two equivalent resonance structures can be drawn for S32-.
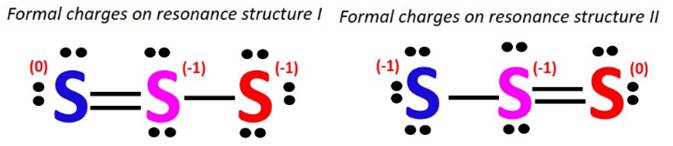
Accompanied by the delocalization of the lone pairs and the pi-bonded electrons, the formal charges also change their positions.
The central S-atom possesses a -1 formal charge in both resonance structures.
The double-bonded S-atom has a zero or no formal charge while there is a -1 formal charge on the single-bonded S-atom as well.
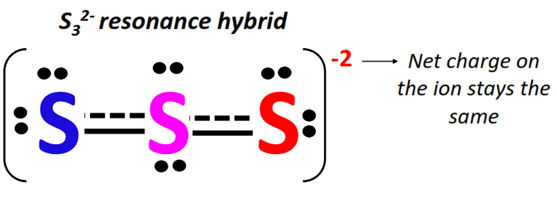
The two resonance contributing forms of S32- are combined using double-headed arrows.
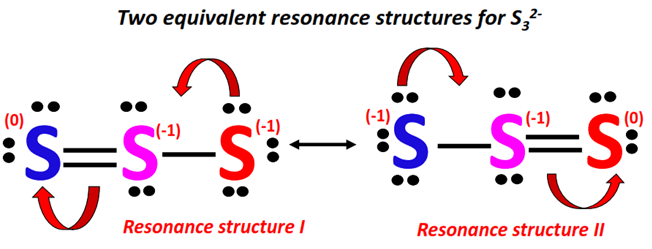
The actual S32- structure is a weighted average of the above resonance forms. Each resonance structure contributes equally to the S32- resonance hybrid.
The pi-bond is thus shown using dotted lines on the S32- resonance hybrid, indicating a delocalized pi-electron cloud.
The S=S bond can be formed between any two S-atoms in S32- and it keeps shifting its position continuously from one side to another on the ion.
Net charge on S32- = -1 + 0 + (-1) = -2
The S32- resonance hybrid is thus enclosed in square brackets and a -2 charge is placed on the top right corner as shown below.