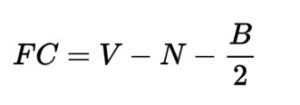How to use pKb to pKa calculator?
Using the above calculator you can easily convert the pKb value to pKa.
- Enter a pKb value in the input field labeled “Enter pKb value =”.
- Click the “Calculate pKa” button.
- The result [pKa] will be displayed in the “result” section with Step by Step solution.
How to calculate pKa from pKb?
The formula that relates pKa to pKb is –
pKa + pKb = pKw …………. Equation (i)
In the above equation, pKw refers to the water dissociation constant.
A water (H2O) molecule undergoes autoionization at room temperature (25°C) to release H+ and OH– ions, as shown below.

The water dissociation constant (Kw) can be determined as per equation (ii).
Kw = [H+][OH–]…………Equation (ii)
Kw has a fixed value at r.t.p i.e., Kw = 1 x 10-14. So, pKw can be calculated by taking the negative logarithm of this value as shown in equation (iii).
pKw = -log10Kw…………. Equation (iii)
pKw = -log10 (1 x 10-14) = 14
Thus, putting the above-determined value of pKw into equation (i) and making pKa the subject of the formula gives us the final equation, which we can use to find pKa if the value of pKb is known.
pKa + pKb = 14
∴ pKa = 14 – pKb

Let’s understand this concept further by practicing with the solved examples given below.
Example of calculating pKa value using “pKb to pKa calculator”
Let’s say we have given the value of pKb and we need to calculate the pKa value.
For example – If the pKb value = 9.23, what is the [pKa] value?
As we know, the formula to convert pKb to pKa is –
∴ pKa = 14 – pKb
So, Just put this value of [pKb] in the calculator field, and press calculates button to get step by step solution.
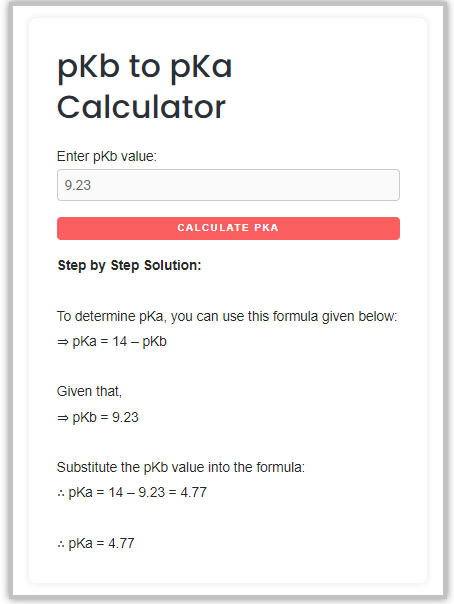
∴ So, the approx pKa value for the given pKb is 4.77.
Numericals based on “Converting pKb value to pKa“
Convert the pKb value of 4.1 to a pKa value. |
To determine pKa, you can use the formula given below: ⇒ pKa = 14 – pKb Given that,
⇒ pKb = 4.1 Substitute the pKb value into the formula: ∴ pKa = 14 – 4.1 = 9.9 ∴ pKa = 9.9 |
What is the pKa for hydrofluoric acid (HF) if the pKb is 11? |
∴ pKa = 14 – pKb Given, pKb = 11
∴ pKa = 14 – 11 ∴ pKa = 3
|