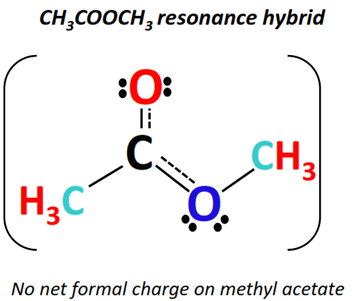
The question is –
How many Lewis resonance structures are possible for methyl acetate (CH3COOCH3)?
Answer:
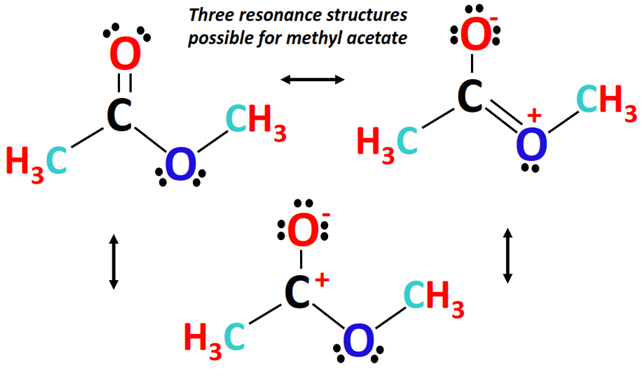
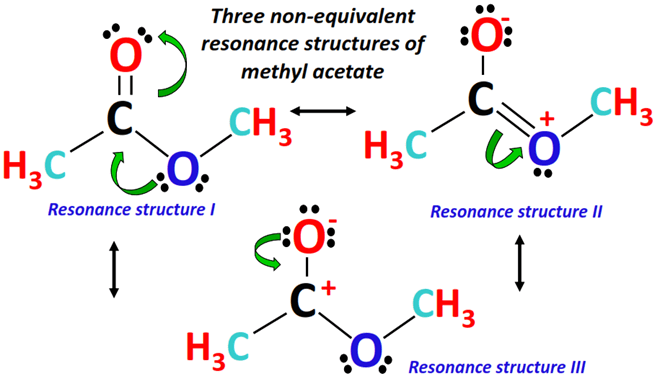
⇒ Three different Lewis resonance structures can possibly be drawn for methyl acetate, as shown below.
Explanation:
Methyl acetate or methyl ethanoate is a carboxylate ester represented by the molecular formula C3H6O2 or CH3COOCH3. The COO represents an ester functional group.
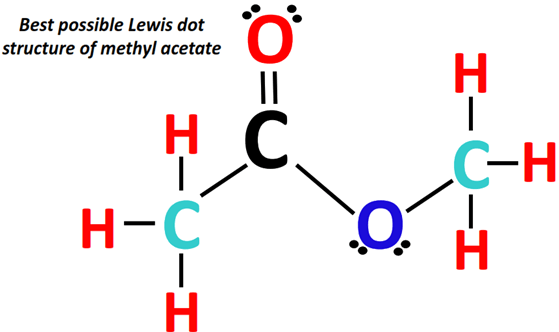
The best possible Lewis structure of methyl acetate has a carbon (C) atom at the center. It is single-bonded to a methyl (-CH3) group on one side and a methoxy (-OCH3) group on the other side.
In contrast, there is an oxygen (O) atom double-covalently bonded to the central C-atom on the third end. It has a total of 30 valence electrons.
An alternate Lewis structure is drawn from the above Lewis structure of methyl acetate, as a lone pair from the single-bonded O-atom (marked blue) shifts to form a double bond between the central C-atom (marked black) and the respective O-atom.
The C=O double bond on the other side of the molecule breaks and an extra lone pair is placed on the O-atom (marked red).
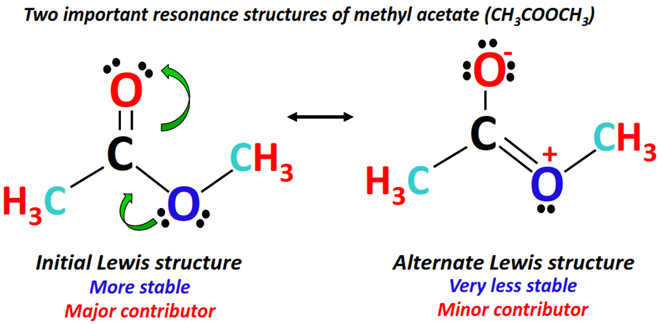
The O-atom gaining an extra lone pair obtains a -1 formal charge while a +1 formal charge appears on the O-atom donating its lone pair.
The above two structures (combined using double-headed arrows) are important resonance-contributing forms of methyl acetate.
Resonance structure I is the most stable so it is a major contributor as opposed to resonance structure II, a minor contributor due to the presence of specific formal charges.
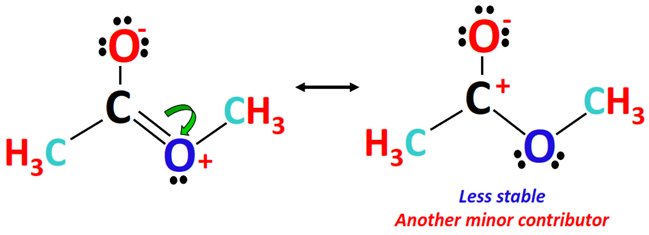
Furthermore, another minor resonance contributing form of methyl acetate can be drawn, as shown below.
Here, both the O-atoms are single-bonded to the central C-atom. The O-atom carrying 3 lone pairs has a -1 formal charge.
There is zero or no formal charge on the O-atom possessing 2 lone pairs while there is a +1 formal charge on the central C-atom in this structure.
The actual methyl acetate molecule is a hybrid of all the above resonance forms.
In terms of stability resonance structure II is the least stable (due to a positive charge on an electronegative O-atom) followed by structure III (negative charge on electronegative O-atom and positive charge on electropositive C-atom) followed by resonance structure I (no formal charges so most stable).
In conclusion, the methyl acetate resonance hybrid represents resonance structure I the most in terms of energy and stability.