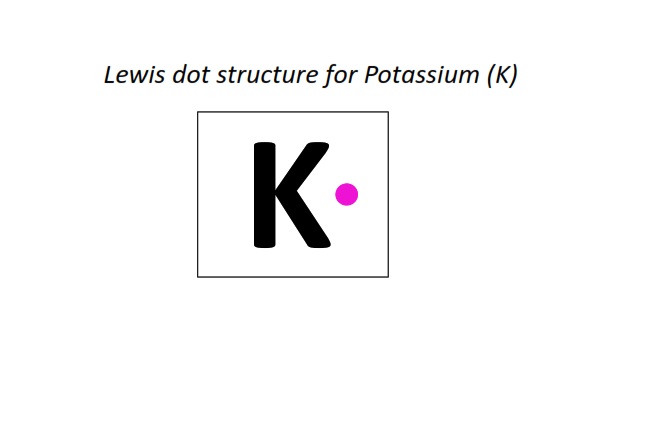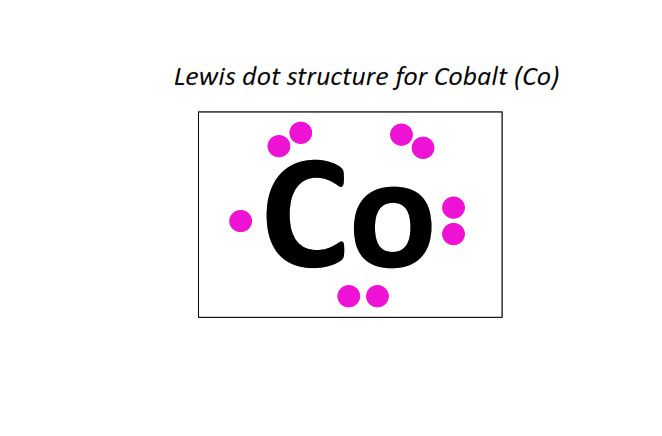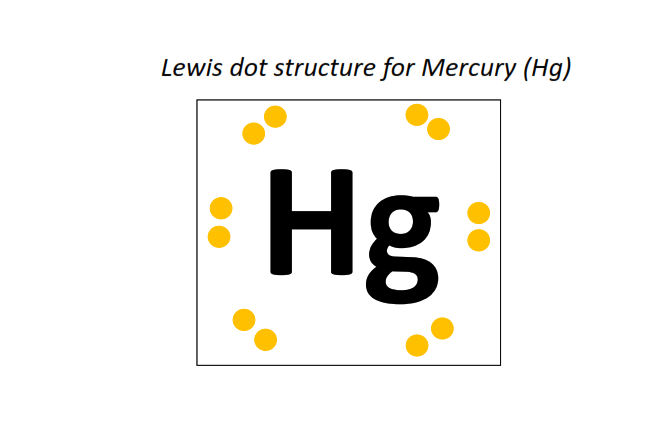List of electron dot structure for all elements of the periodic table | Diagram |
Lewis structure is a simplified representation of all the valence electrons of an atom or a molecule. Valence electrons are the electrons present in the outermost shell of an atom and those that get involved in chemical bonding.
Here is the list of all elements (1 to 118) in the periodic table with their Lewis dot structure also called electron dot structure.
Lewis dot structure for all elements (1-118)
Atomic no. | Lewis dot structure for all elements |
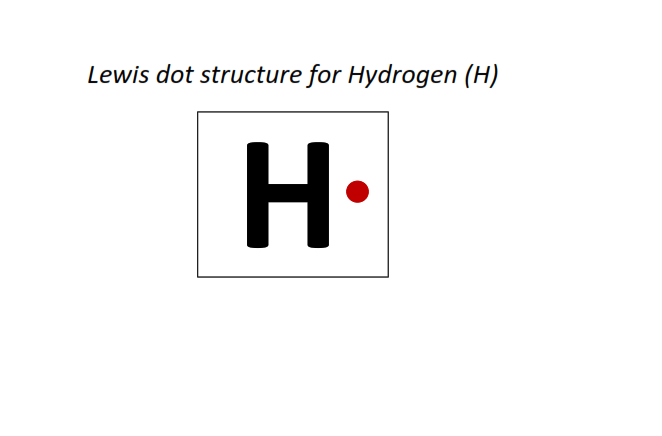
1 | Lewis dot structure of Hydrogen (H)[1]
|
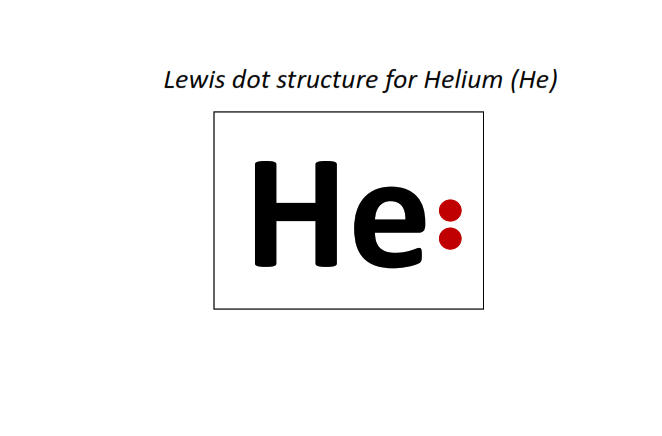
2 | Lewis dot structure of Helium (He)[2]
|
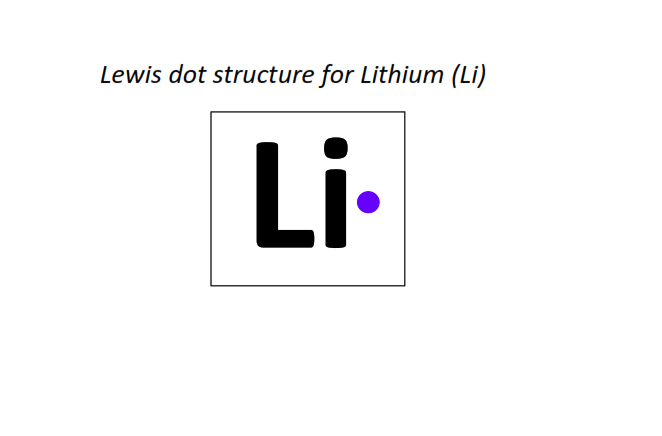
3 | Lewis dot structure of Lithium (Li)[2, 1]
|
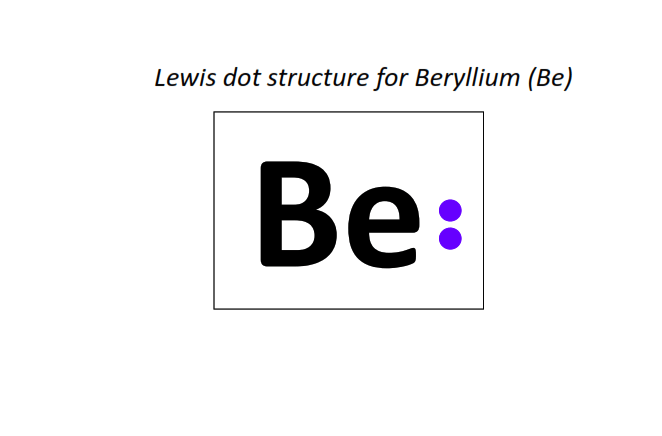
4 | Lewis dot structure of Beryllium (Be)[2, 2]
|
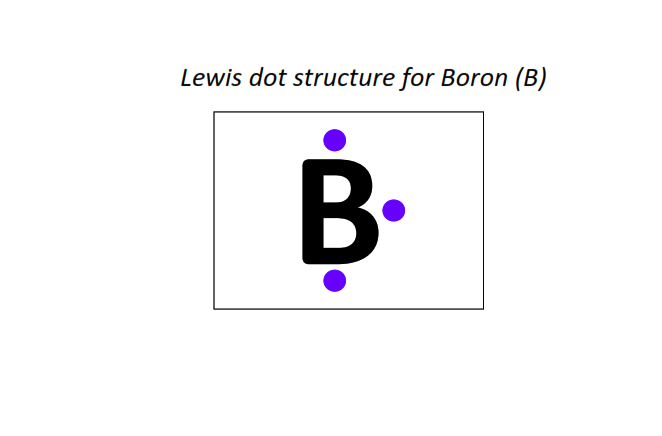
5 | Lewis dot structure of Boron (B)[2, 3]
|
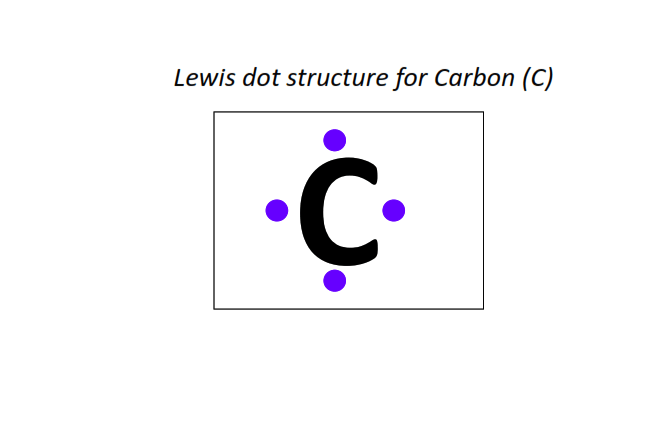
6 | Lewis dot structure of Carbon (C)[2, 4]
|
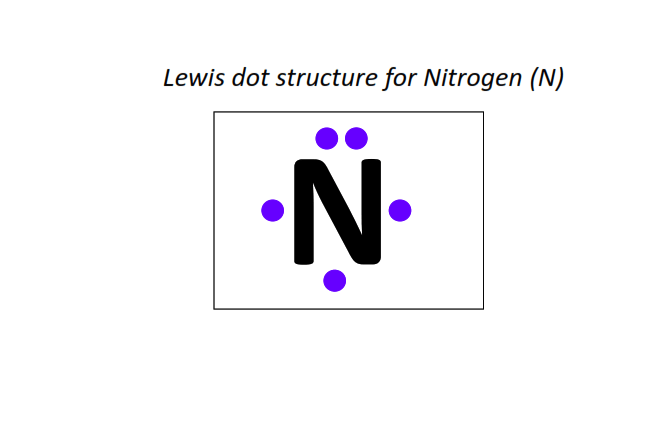
7 | Lewis dot structure of Nitrogen (N)[2, 5]
|
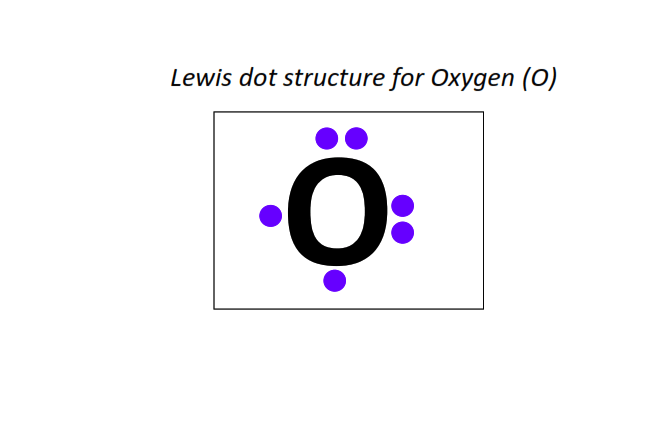
8 | Lewis dot structure of Oxygen (O)[2, 6]
|
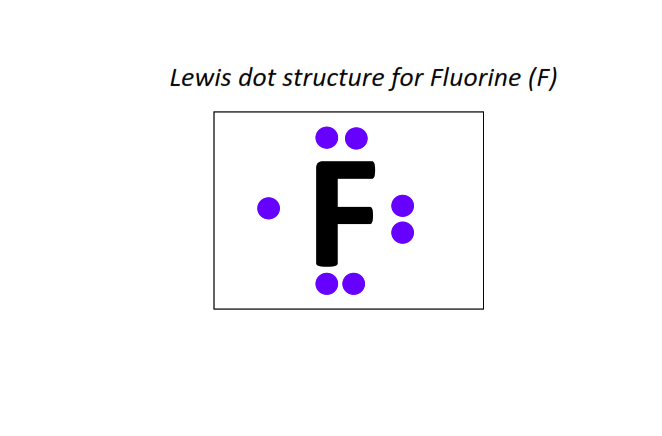
9 | Lewis dot structure of Fluorine (F)[2, 7]
|
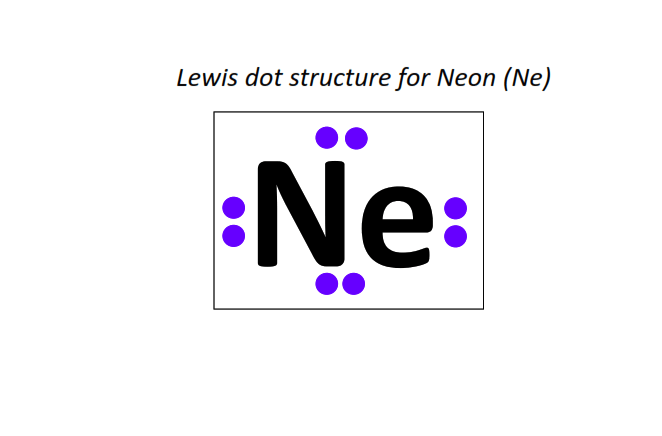
10 | Lewis dot structure of Neon (Ne)[2, 8]
|
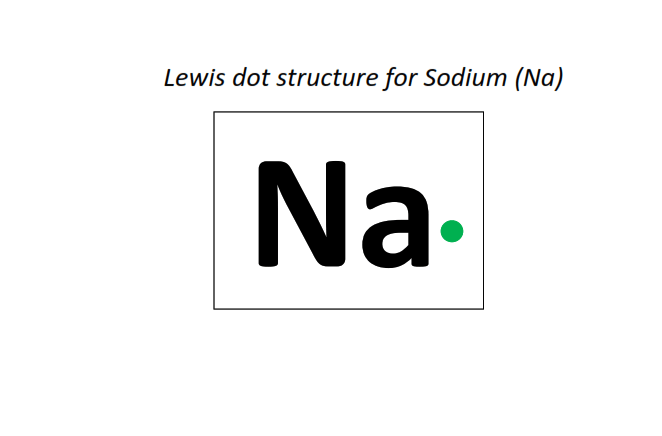
11 | Lewis dot structure of Sodium (Na)[2, 8, 1]
|
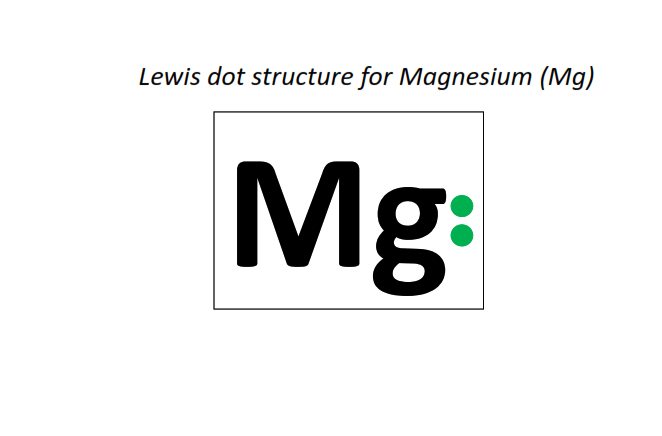
12 | Lewis dot structure of Magnesium (Mg)[2, 8, 2]
|
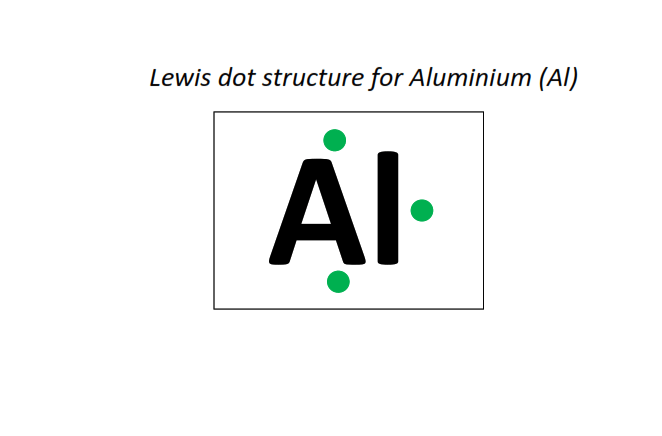
13 | Lewis dot structure of Aluminum (Al)[2, 8, 3]
|
14 | Lewis dot structure of Silicon (Si)[2, 8, 4]
|
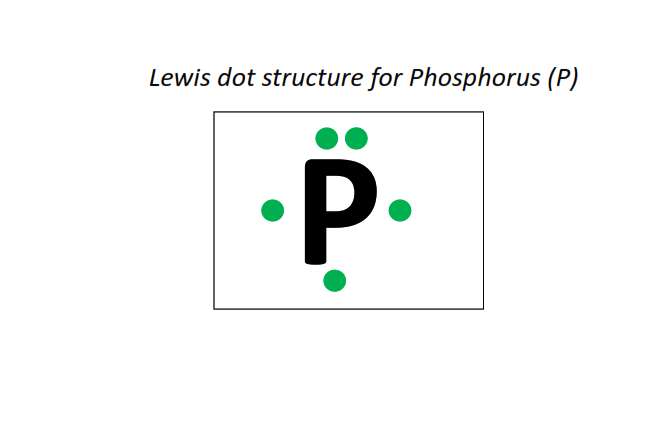
15 | Lewis dot structure of Phosphorus (P)[2, 8, 5]
|
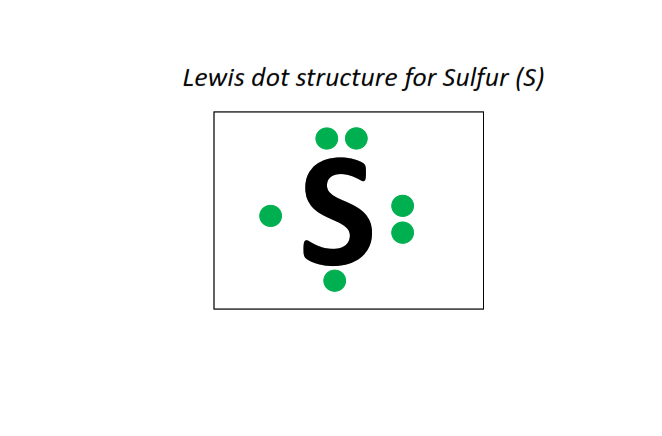
16 | Lewis dot structure of Sulfur (S)[2, 8, 6]
|
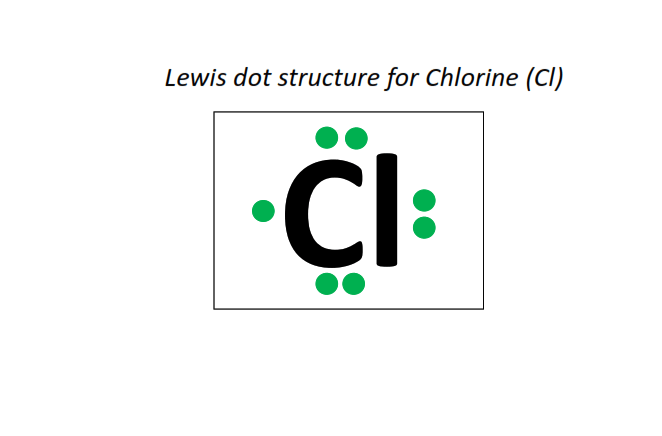
17 | Lewis dot structure of Chlorine (Cl)[2, 8, 7]
|
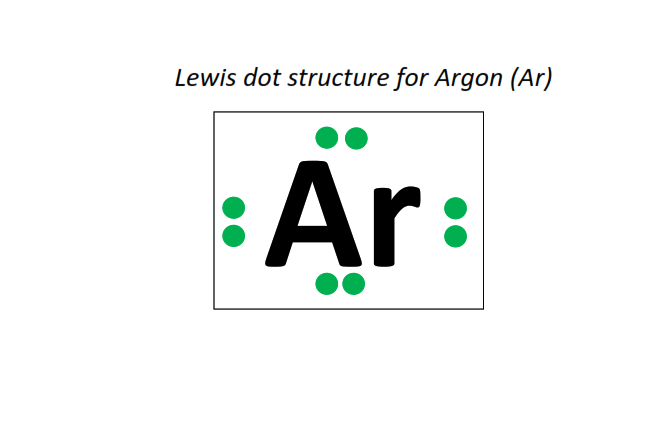
18 | Lewis dot structure of Argon (Ar)[2, 8, 8]
|
19 | Lewis dot structure of Potassium (K)[2, 8, 8, 1]
|
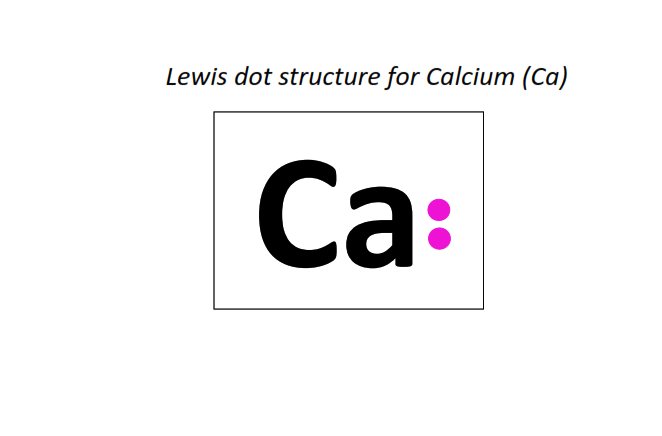
20 | Lewis dot structure of Calcium (Ca)[2, 8, 8, 2]
|
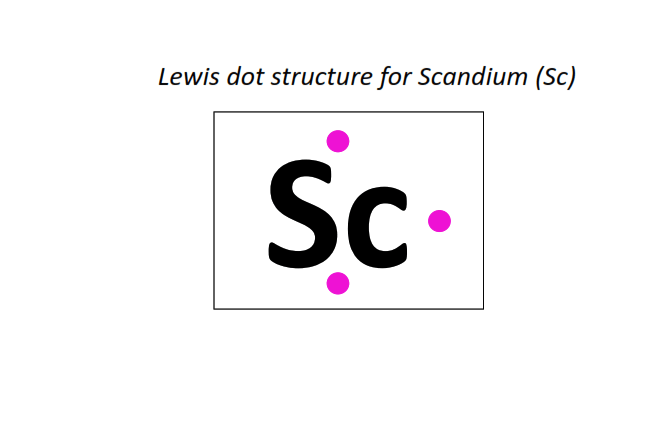
21 | Lewis dot structure of Scandium (Sc)[2, 8, 9, 2]
|
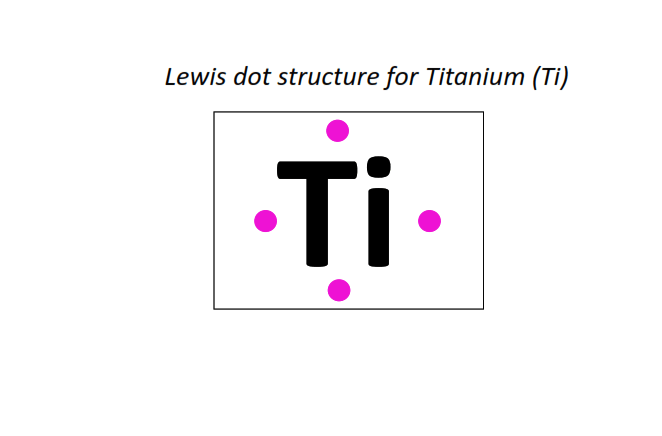
22 | Lewis dot structure of Titanium (Ti)[2, 8, 10, 2]
|
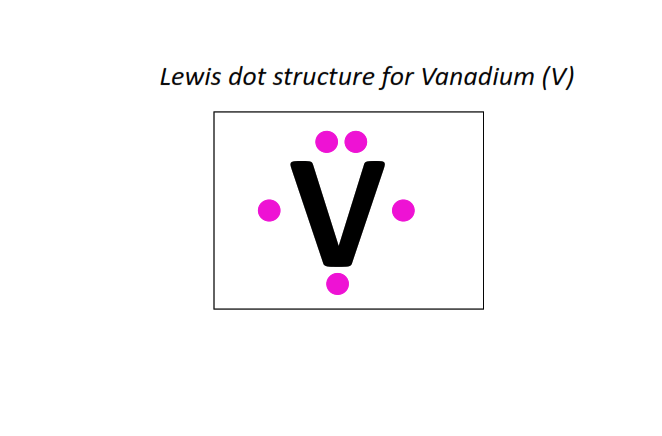
23 | Lewis dot structure of Vanadium (V)[2, 8, 11, 2]
|
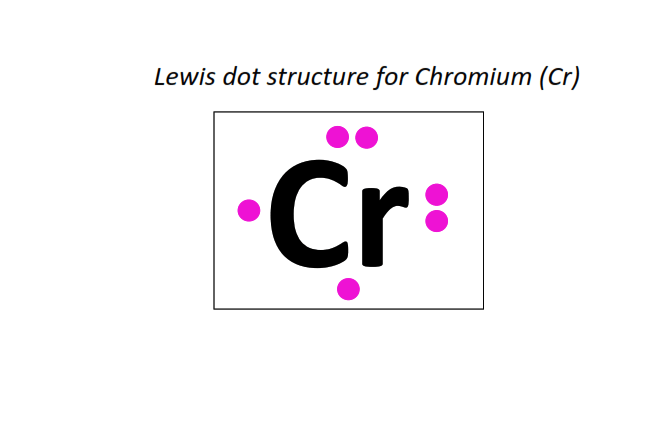
24 | Lewis dot structure of Chromium (Cr)[2, 8, 13, 1]
|
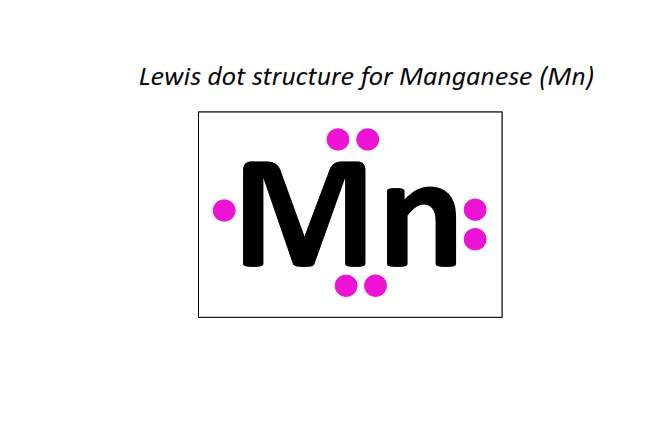
25 | Lewis dot structure of Manganese (Mn)[2, 8, 13, 2]
|
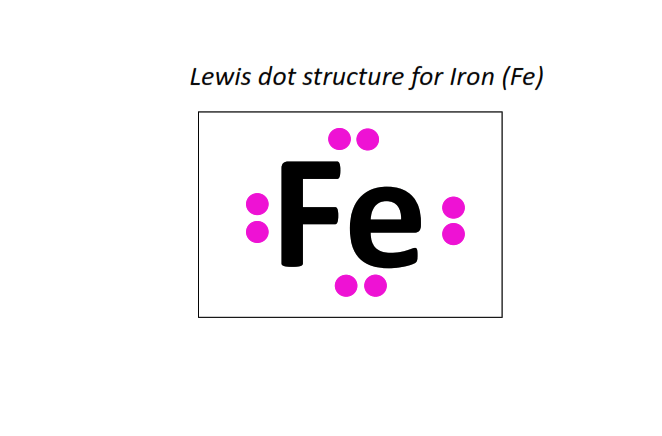
26 | Lewis dot structure of Iron (Fe)[2, 8, 14, 2]
|
27 | Lewis dot structure of Cobalt (Co)[2, 8, 15, 2]
|
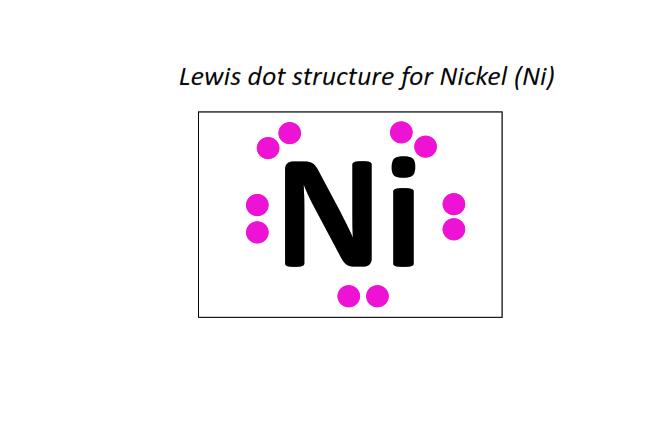
28 | Lewis dot structure of Nickel (Ni)[2, 8, 16, 2]
|
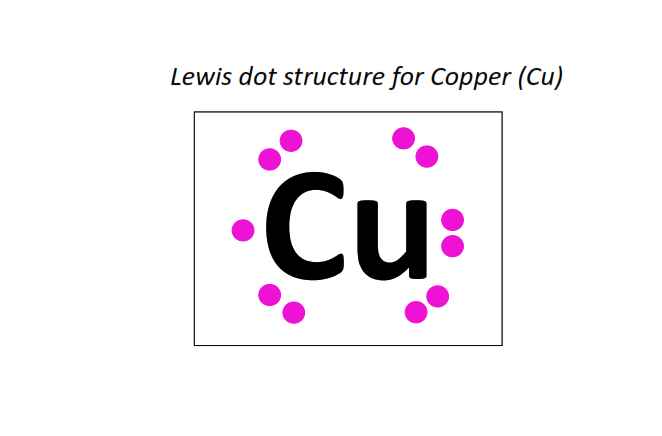
29 | Lewis dot structure of Copper (Cu)[2, 8, 18, 1]
|
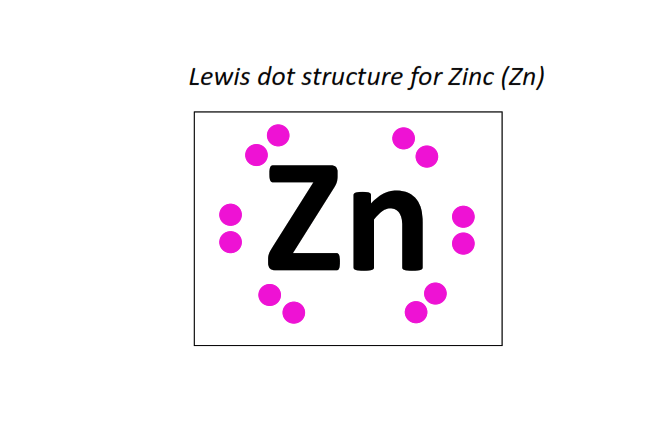
30 | Lewis dot structure of Zinc (Zn)[2, 8, 18, 2]
|
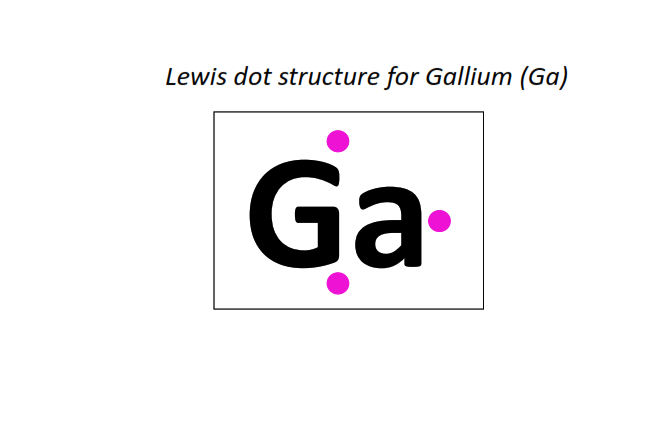
31 | Lewis dot structure of Gallium (Ga)[2, 8, 18, 3]
|
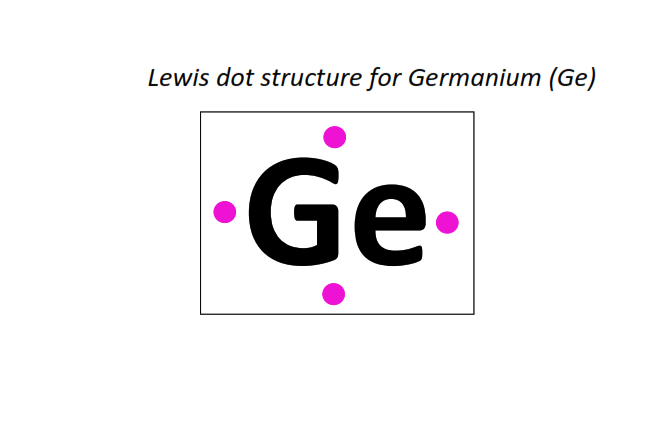
32 | Lewis dot structure of Germanium (Ge)[2, 8, 18, 4]
|
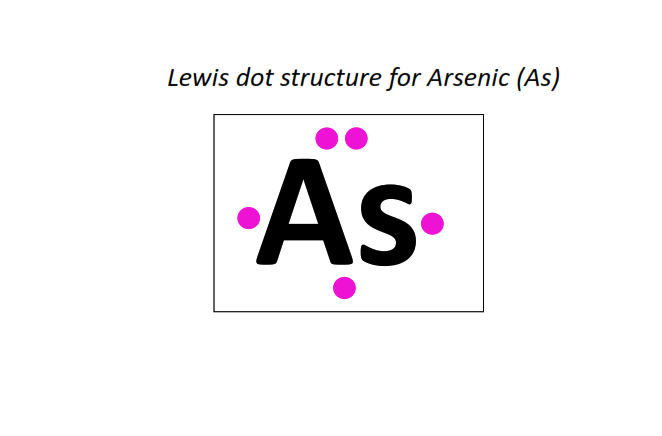
33 | Lewis dot structure of Arsenic (As)[2, 8, 18, 5]
|
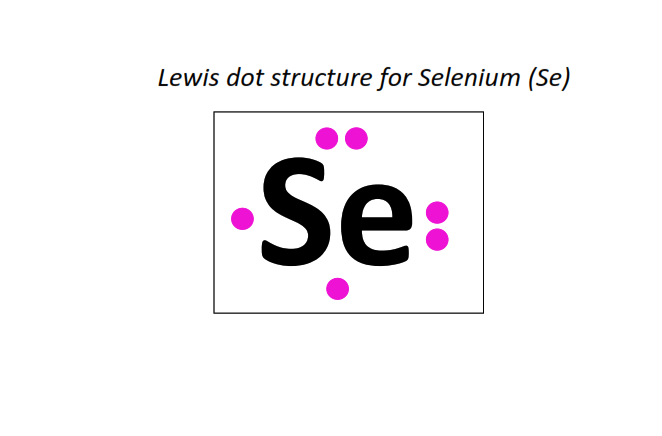
34 | Lewis dot structure of Selenium (Se)[2, 8, 18, 6]
|
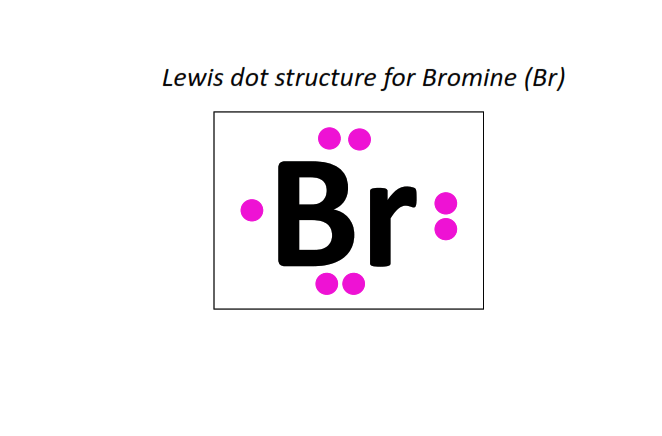
35 | Lewis dot structure of Bromine (Br)[2, 8, 18, 7]
|
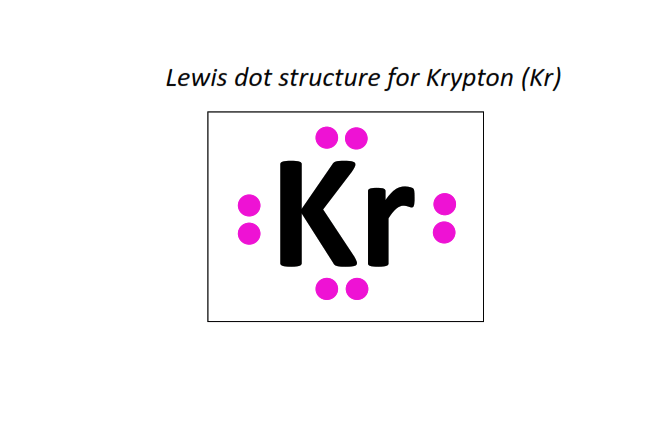
36 | Lewis dot structure of Krypton (Kr)[2, 8, 18, 8]
|
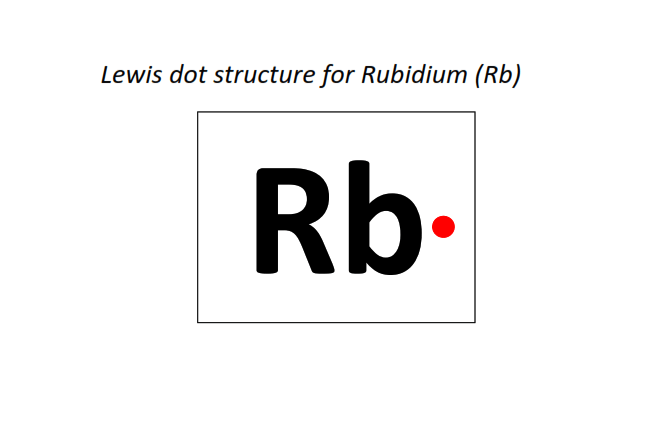
37 | Lewis dot structure of Rubidium (Rb)[2, 8, 18, 8, 1]
|
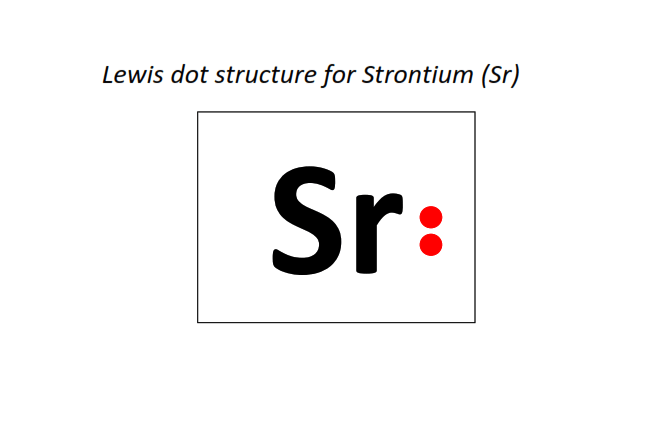
38 | Lewis dot structure of Strontium (Sr)[2, 8, 18, 8, 2]
|
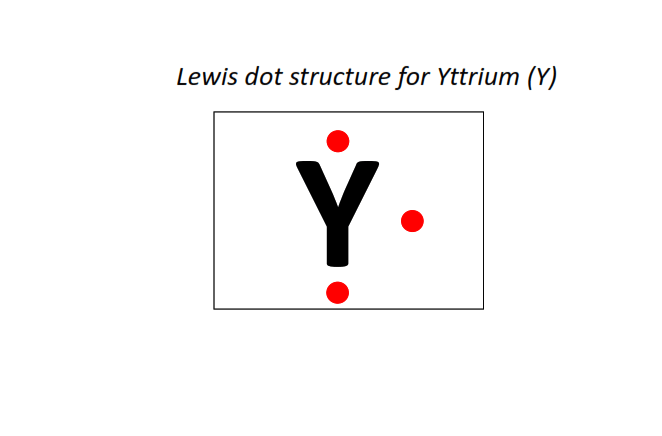
39 | Lewis dot structure of Yttrium (Y)[2, 8, 18, 9, 2]
|
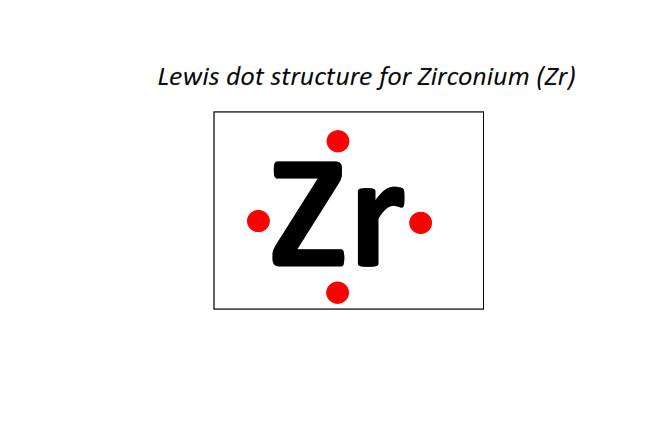
40 | Lewis dot structure of Zirconium (Zr)[2, 8, 18, 10, 2]
|
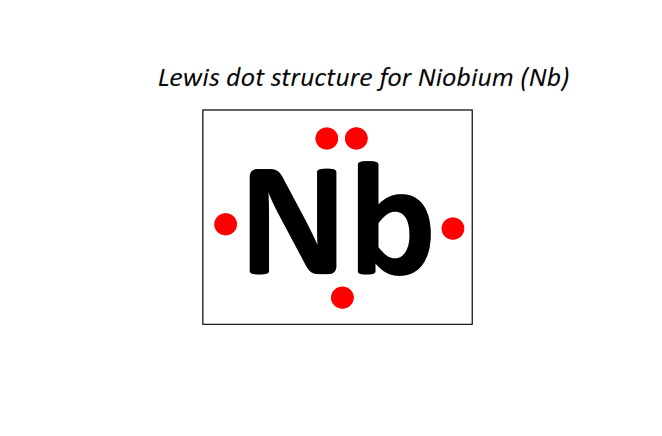
41 | Lewis dot structure of Niobium (Nb)[2, 8, 18, 12, 1]
|
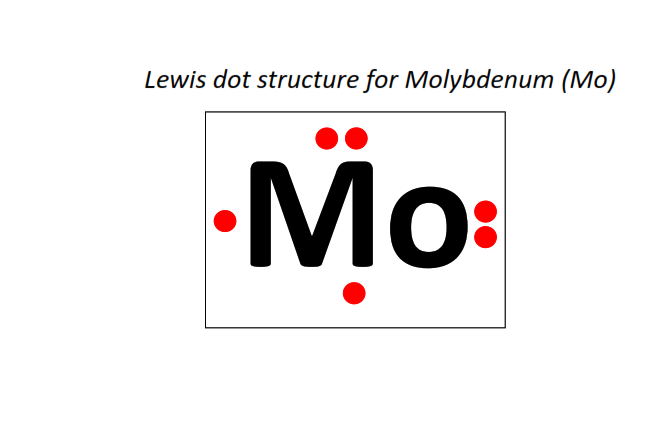
42 | Lewis dot structure of Molybdenum (Mo)[2, 8, 18, 13, 1]
|
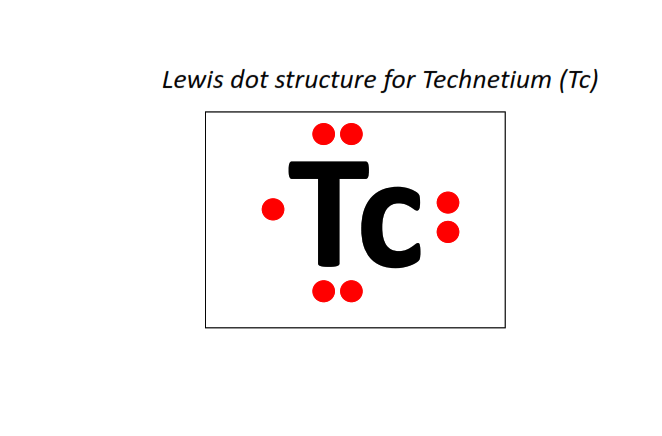
43 | Lewis dot structure of Technetium (Tc)[2, 8, 18, 13, 2]
|
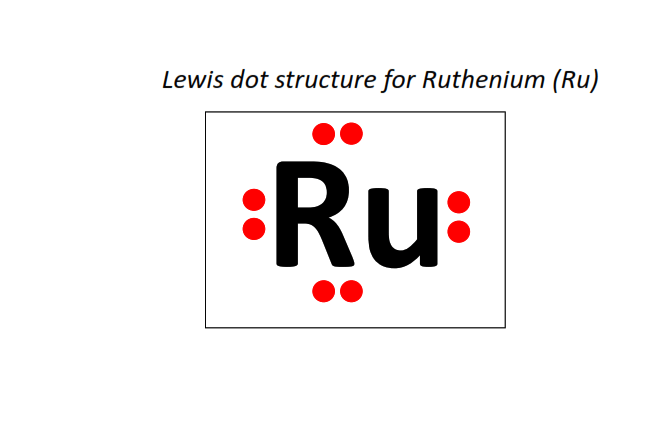
44 | Lewis dot structure of Ruthenium (Ru)[2, 8, 18, 15, 1]
|
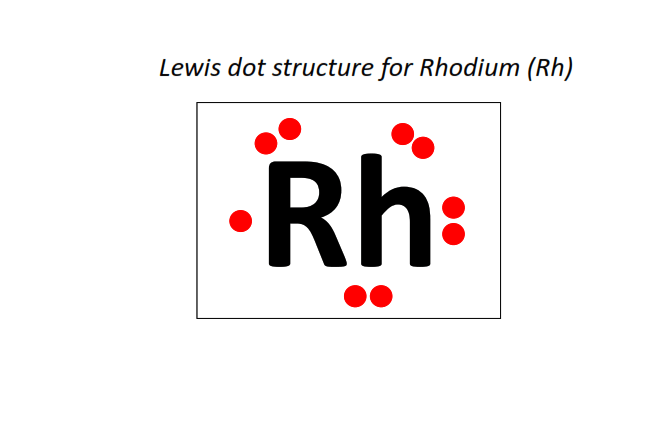
45 | Lewis dot structure of Rhodium (Rh)[2, 8, 18, 16, 1]
|
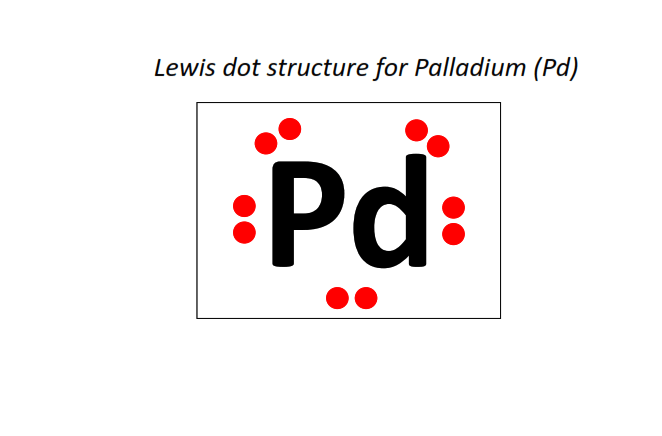
46 | Lewis dot structure of Palladium (Pd)[2, 8, 18, 18]
|
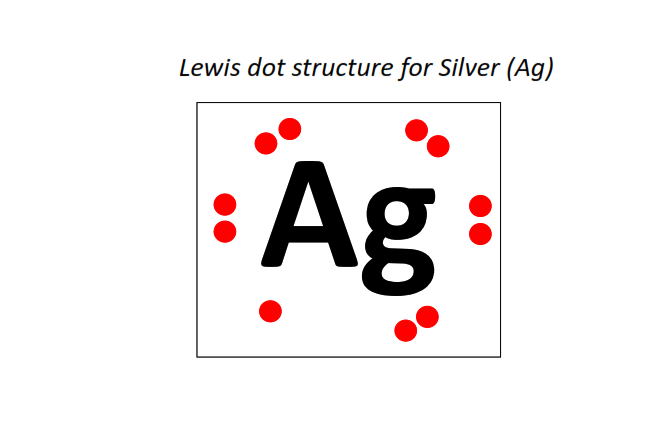
47 | Lewis dot structure of Silver (Ag)[2, 8, 18, 18, 1]
|
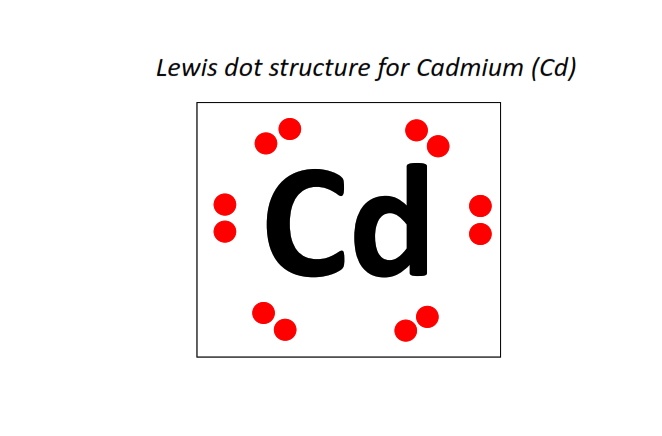
48 | Lewis dot structure of Cadmium (Cd)[2, 8, 18, 18, 2]
|
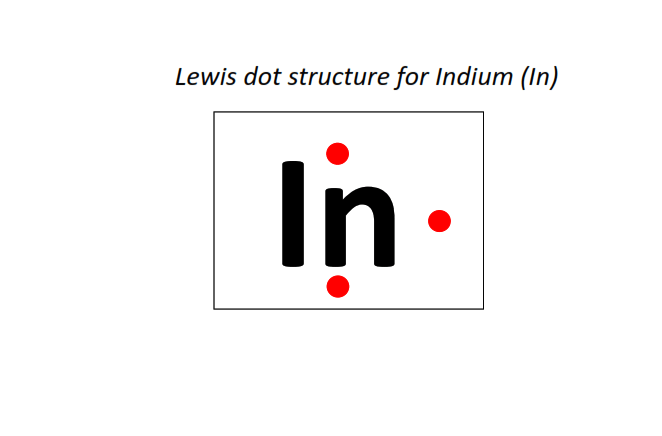
49 | Lewis dot structure of Indium (In)[2, 8, 18, 18, 3]
|
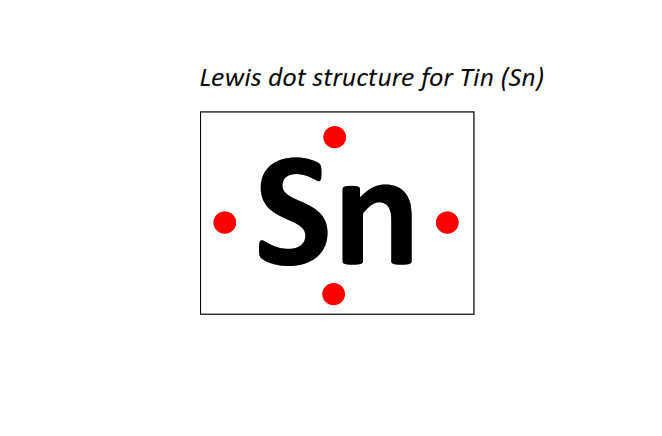
50 | Lewis dot structure of Tin (Sn)[2, 8, 18, 18, 4]
|
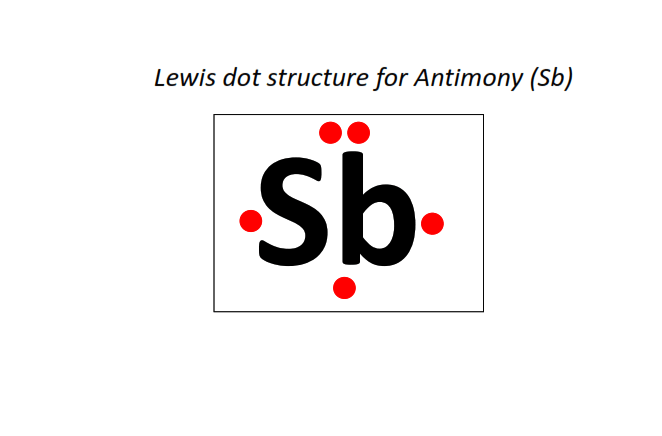
51 | Lewis dot structure of Antimony (Sb)[2, 8, 18, 18, 5]
|
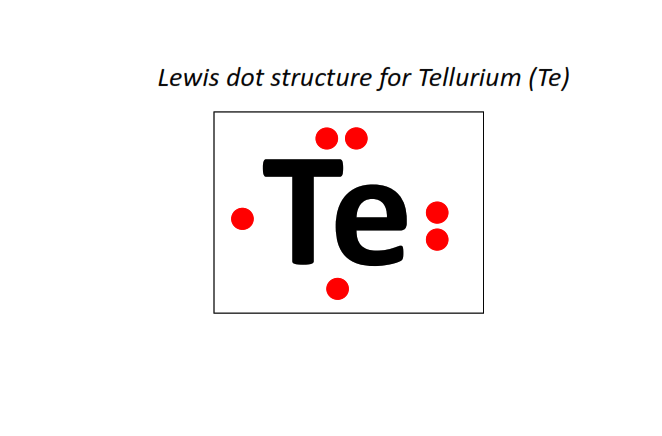
52 | Lewis dot structure of Tellurium (Te)[2, 8, 18, 18, 6]
|
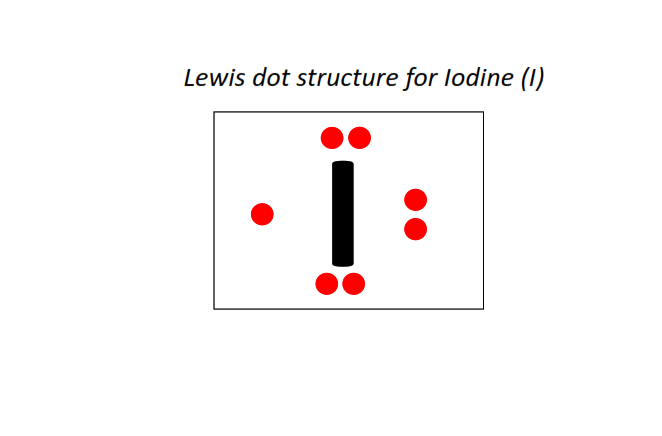
53 | Lewis dot structure of Iodine (I)[2, 8, 18, 18, 7]
|
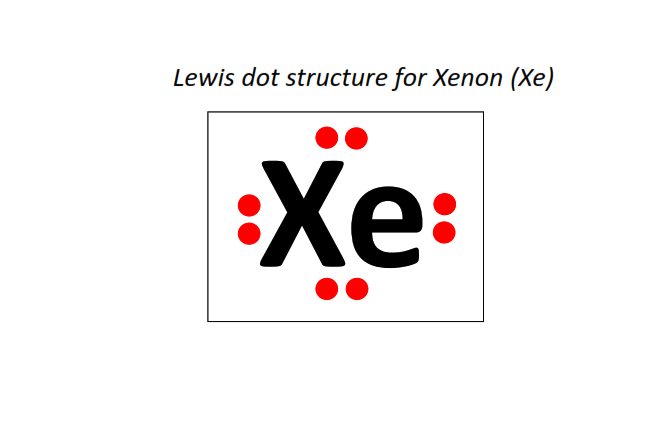
54 | Lewis dot structure of Xenon (Xe)[2, 8, 18, 18, 8]
|
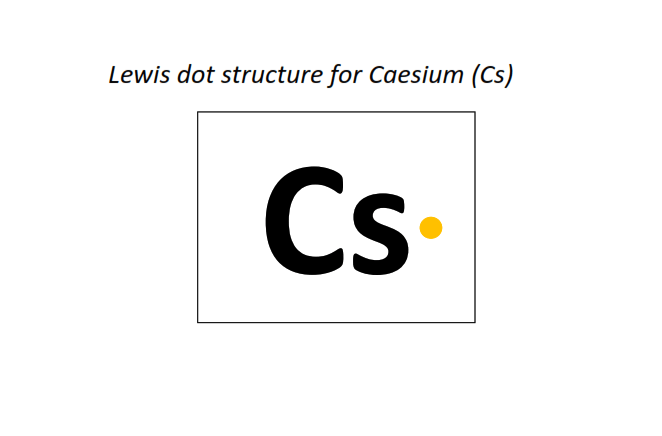
55 | Lewis dot structure of Cesium (Cs)[2, 8, 18, 18, 8, 1]
|
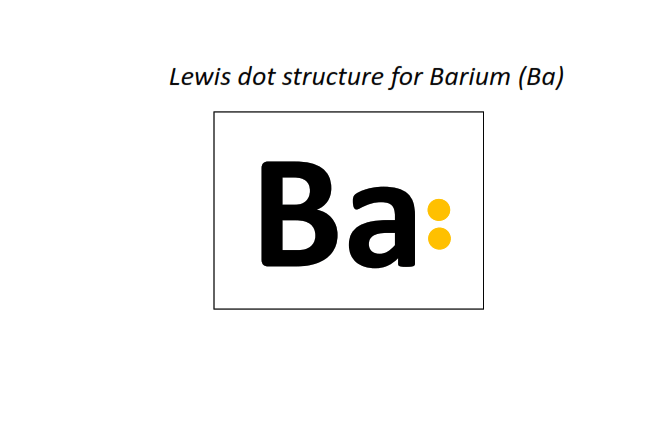
56 | Lewis dot structure of Barium (Ba)[2, 8, 18, 18, 8, 2]
|
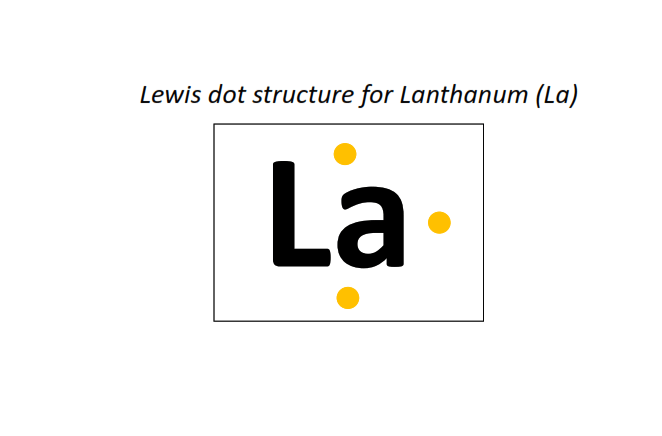
57 | Lewis dot structure of Lanthanum (La)[2, 8, 18, 18, 9, 2]
|
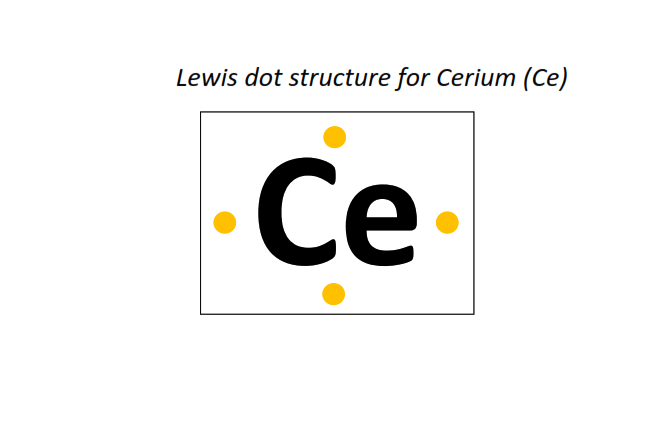
58 | Lewis dot structure of Cerium (Ce)[2, 8, 18, 19, 9, 2]
|
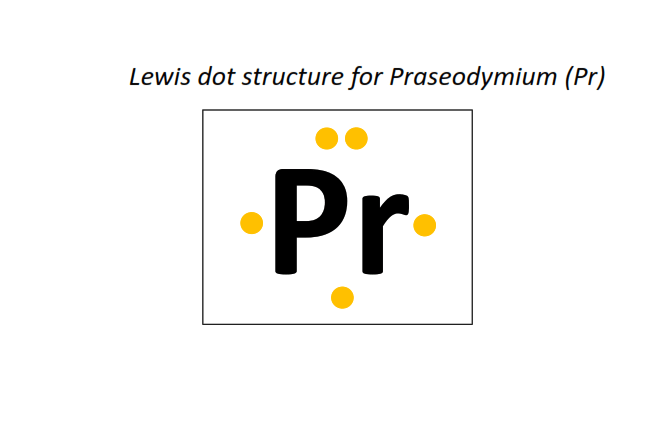
59 | Lewis dot structure of Praseodymium (Pr)[2, 8, 18, 21, 8, 2]
|
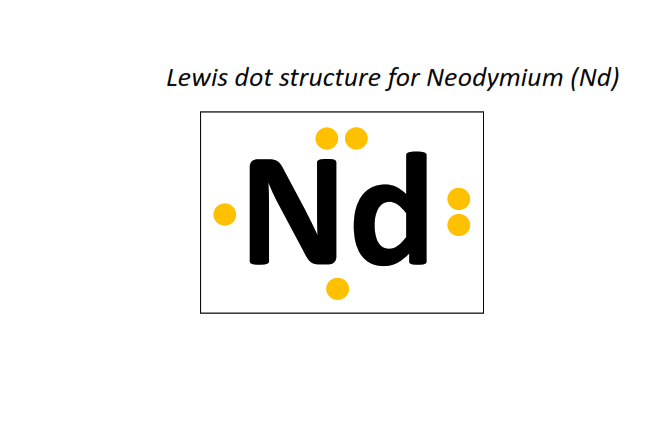
60 | Lewis dot structure of Neodymium (Nd)[2, 8, 18, 22, 8, 2]
|
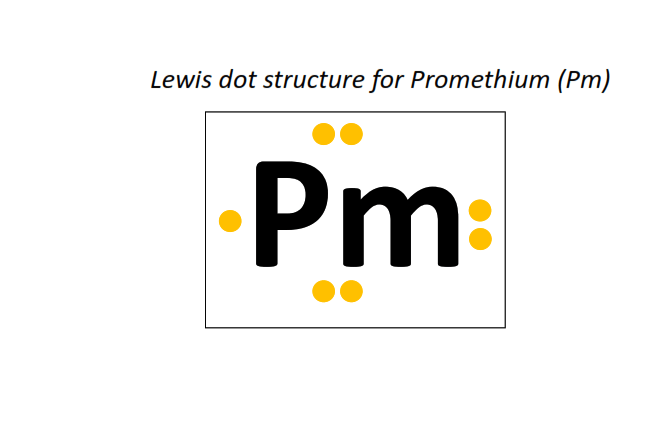
61 | Lewis dot structure of Promethium (Pm)[2, 8, 18, 23, 8, 2]
|
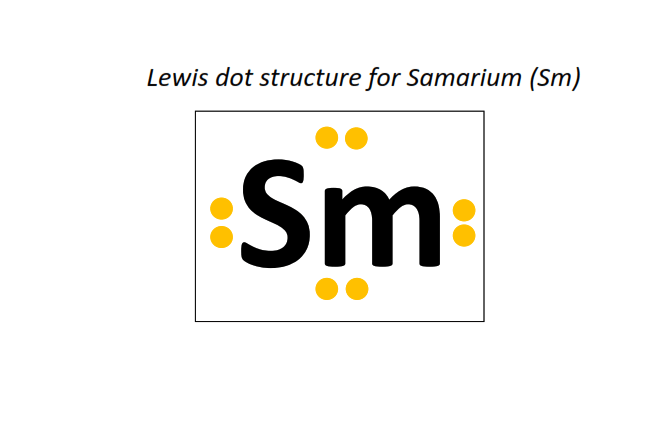
62 | Lewis dot structure of Samarium (Sm)[2, 8, 18, 24, 8, 2]
|
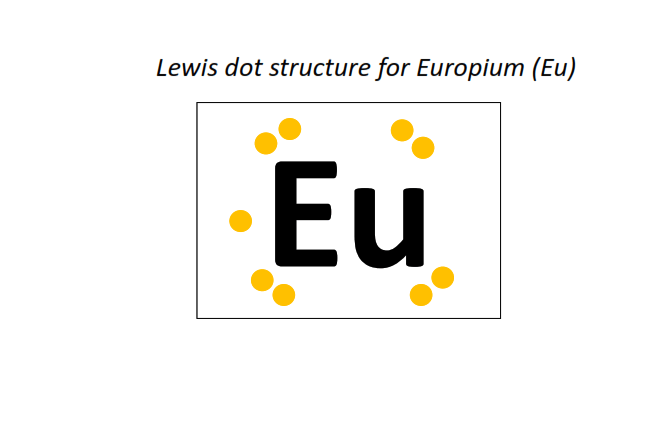
63 | Lewis dot structure of Europium (Eu)[2, 8, 18, 25, 8, 2]
|
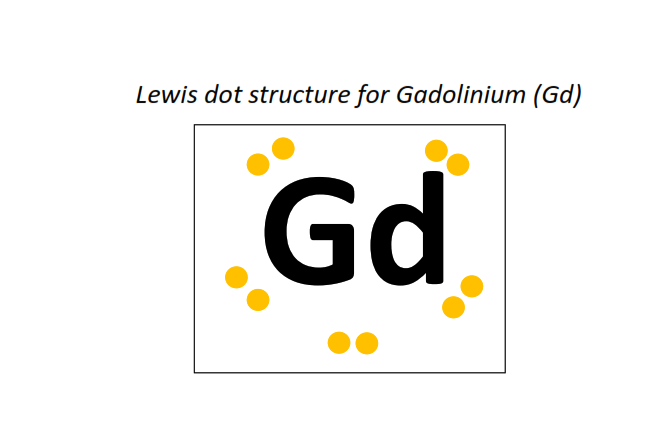
64 | Lewis dot structure of Gadolinium (Gd)[2, 8, 18, 25, 9, 2]
|
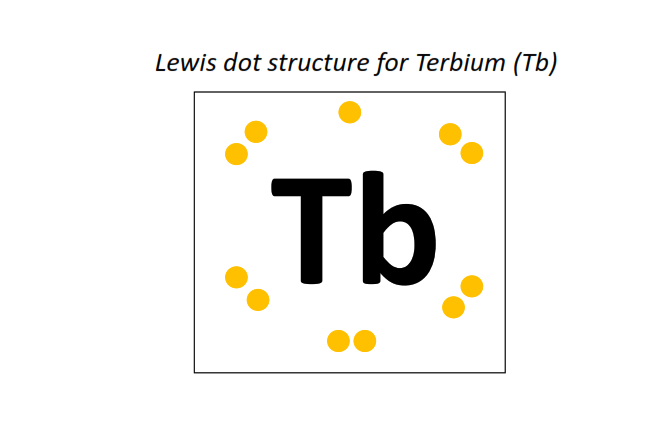
65 | Lewis dot structure of Terbium (Tb)[2, 8, 18, 27, 8, 2]
|
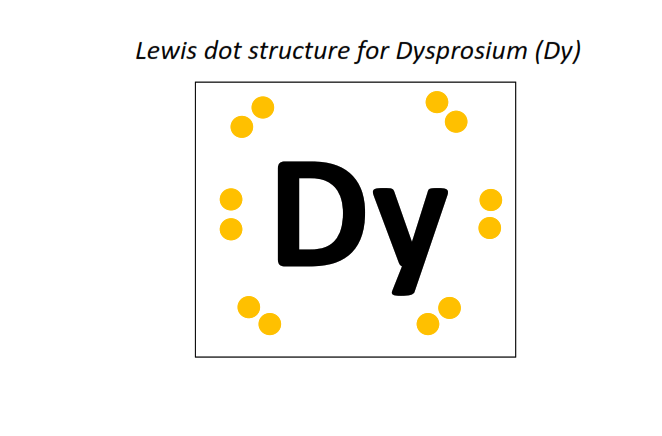
66 | Lewis dot structure of Dysprosium (Dy)[2, 8, 18, 28, 8, 2]
|
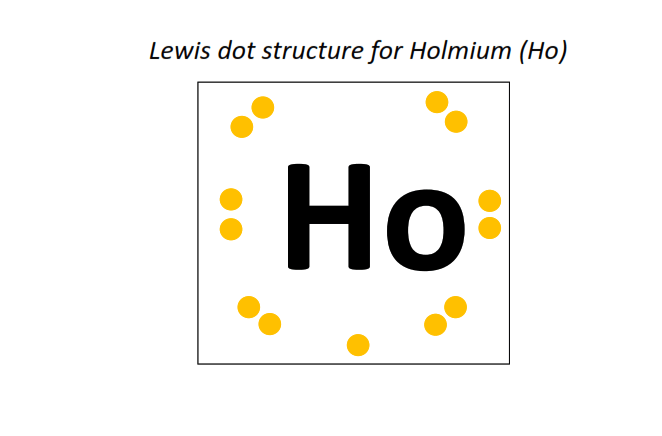
67 | Lewis dot structure of Holmium (Ho)[2, 8, 18, 29, 8, 2]
|
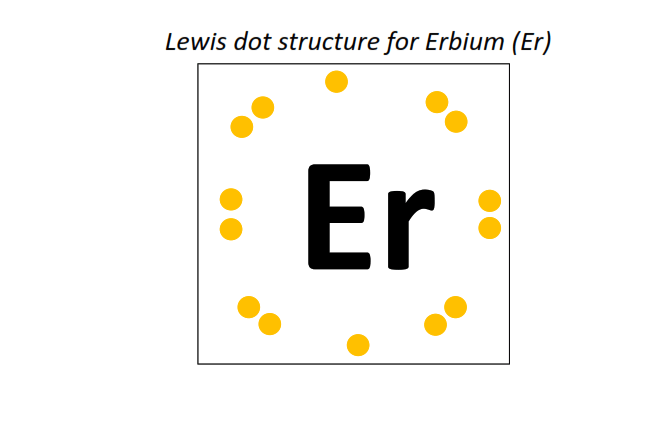
68 | Lewis dot structure of Erbium (Er)[2, 8, 18, 30, 8, 2]
|
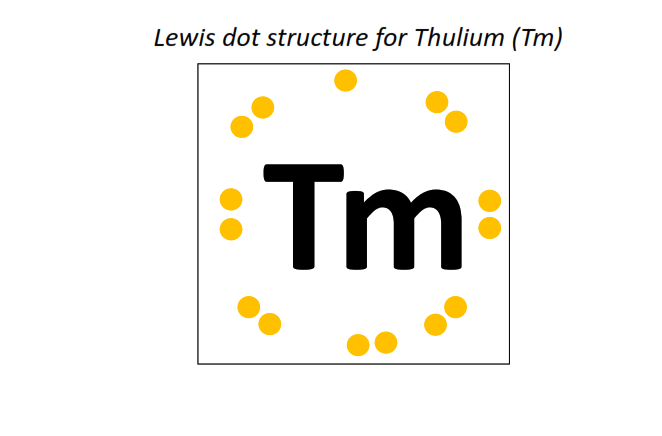
69 | Lewis dot structure of Thulium (Tm)[2, 8, 18, 31, 8, 2]
|
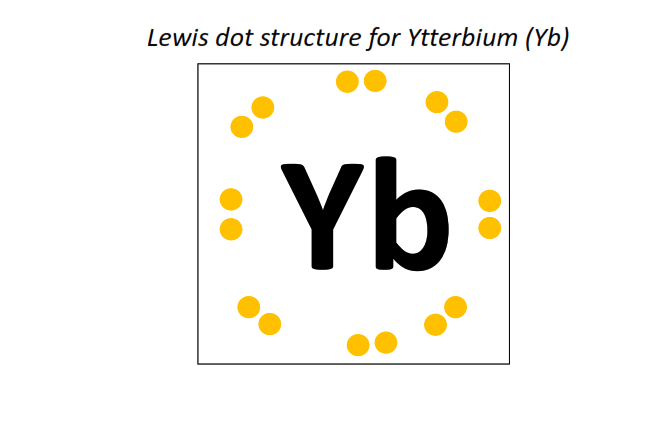
70 | Lewis dot structure of Ytterbium (Yb)[2, 8, 18, 32, 8, 2]
|
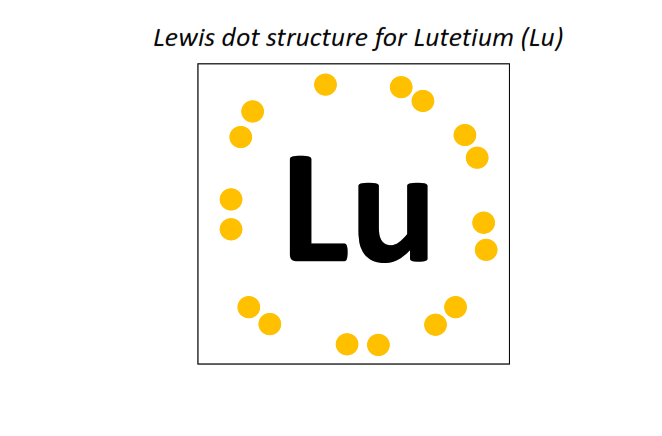
71 | Lewis dot structure of Lutetium (Lu)[2, 8, 18, 32, 9, 2]
|
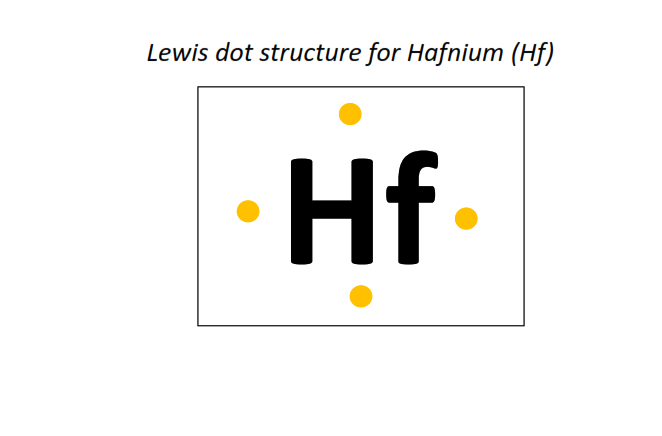
72 | Lewis dot structure of Hafnium (Hf)[2, 8, 18, 32, 10, 2]
|
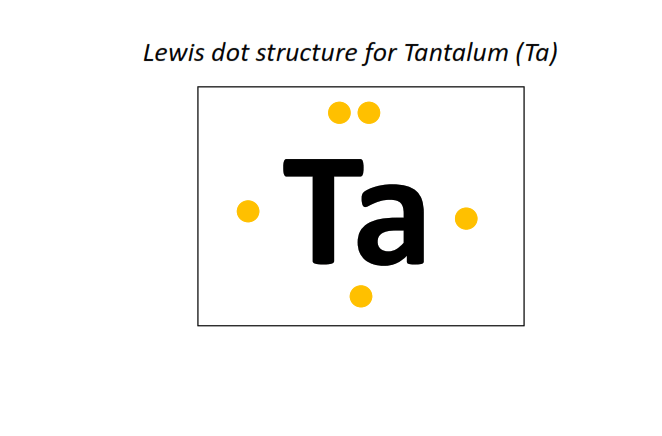
73 | Lewis dot structure of Tantalum (Ta)[2, 8, 18, 32, 11, 2]
|
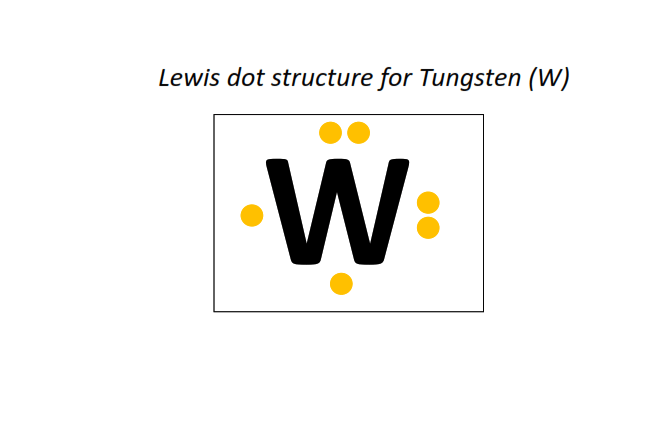
74 | Lewis dot structure of Tungsten (W)[2, 8, 18, 32, 12, 2]
|
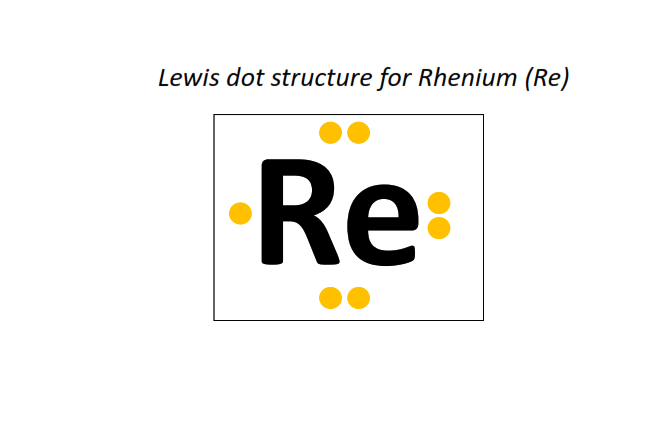
75 | Lewis dot structure of Rhenium (Re)[2, 8, 18, 32, 13, 2]
|
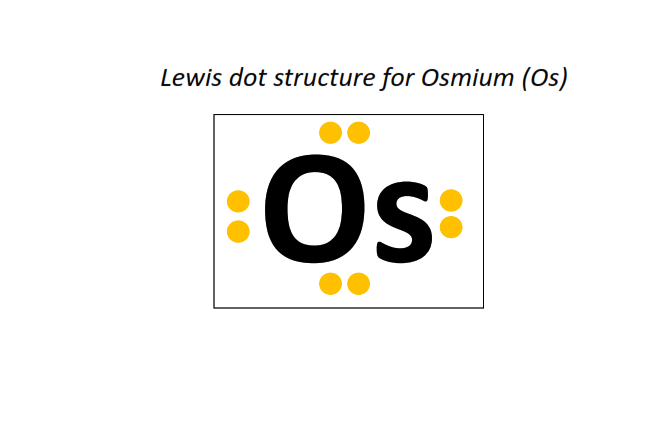
76 | Lewis dot structure of Osmium (Os)[2, 8, 18, 32, 14, 2]
|
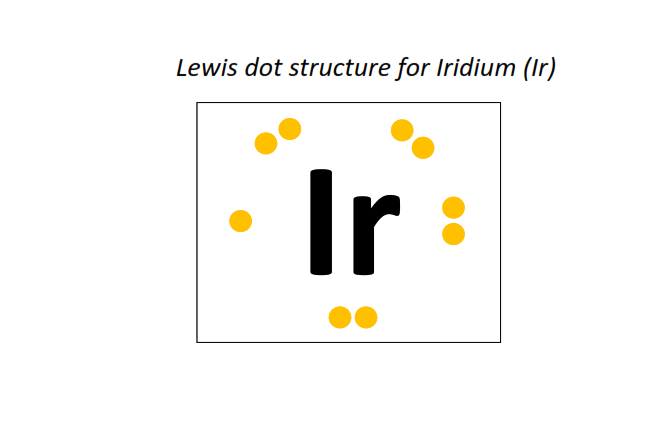
77 | Lewis dot structure of Iridium (Ir)[2, 8, 18, 32, 15, 2]
|
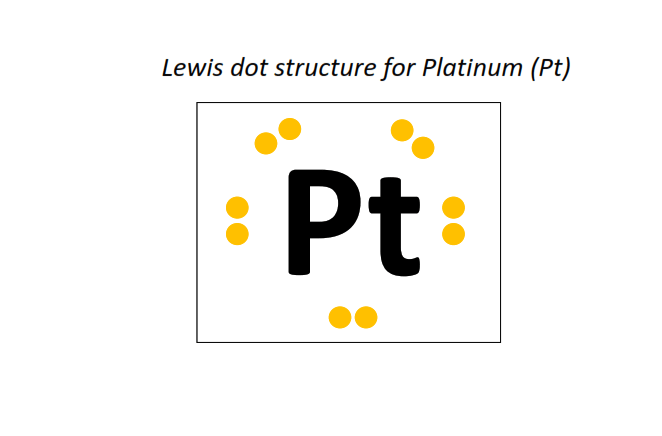
78 | Lewis dot structure of Platinum (Pt)[2, 8, 18, 32, 17, 1]
|
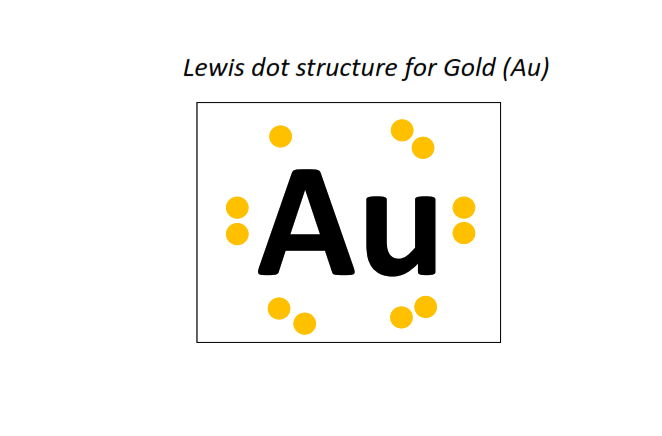
79 | Lewis dot structure of Gold (Au)[2, 8, 18, 32, 18, 1]
|
80 | Lewis dot structure of Mercury (Hg)[2, 8, 18, 32, 18, 2]
|
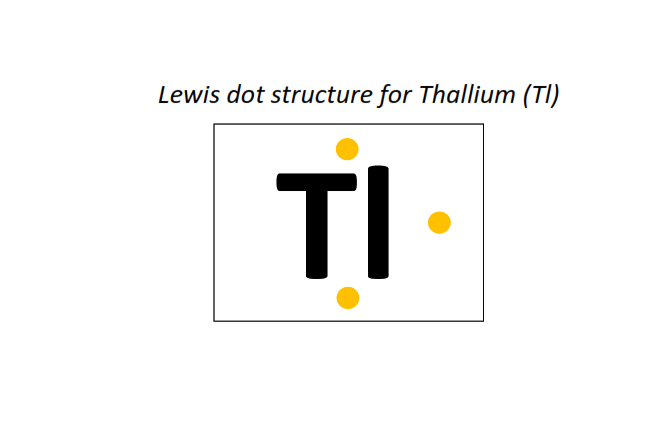
81 | Lewis dot structure of Thallium (Tl)[2, 8, 18, 32, 18, 3]
|
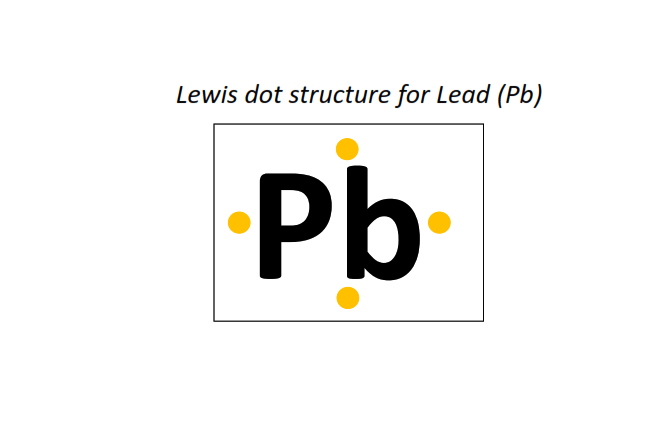
82 | Lewis dot structure of Lead (Pb)[2, 8, 18, 32, 18, 4]
|
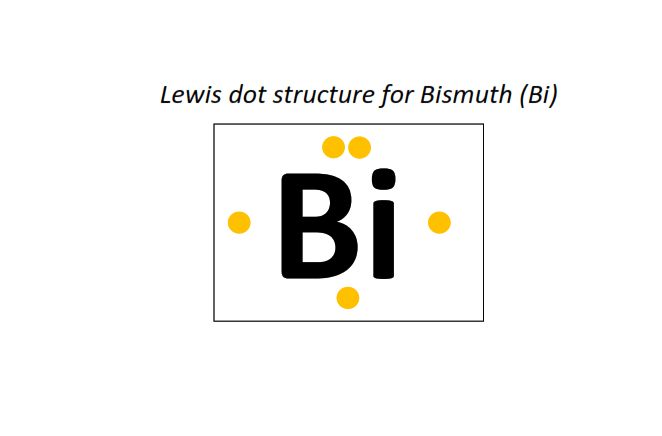
83 | Lewis dot structure of Bismuth (Bi)[2, 8, 18, 32, 18, 5]
|
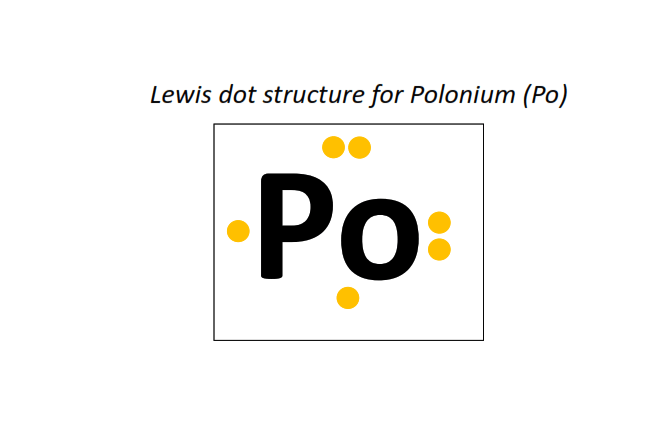
84 | Lewis dot structure of Polonium (Po)[2, 8, 18, 32, 18, 6]
|
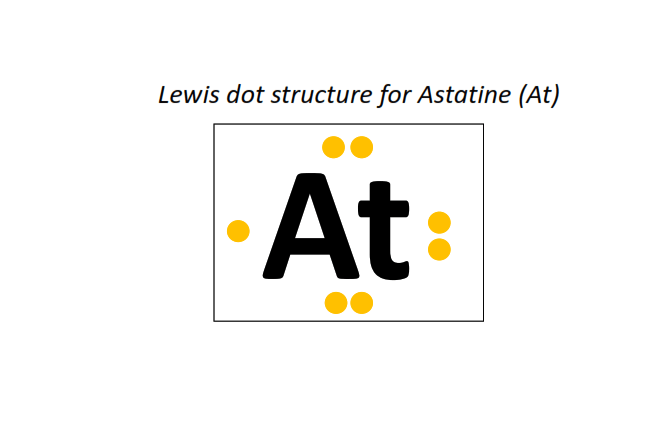
85 | Lewis dot structure of Astatine (At)[2, 8, 18, 32, 18, 7]
|
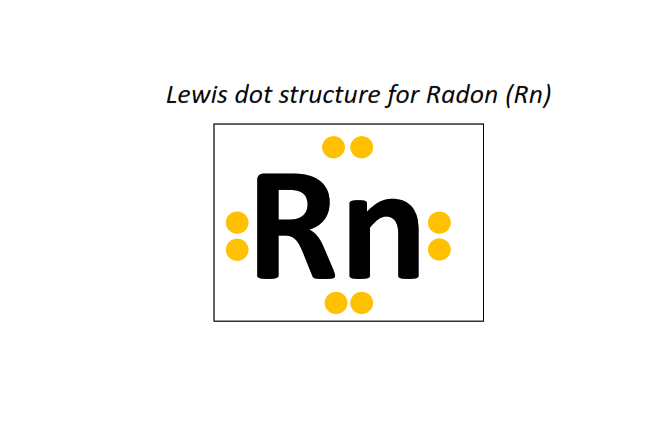
86 | Lewis dot structure of Radon (Rn)[2, 8, 18, 32, 18, 8]
|
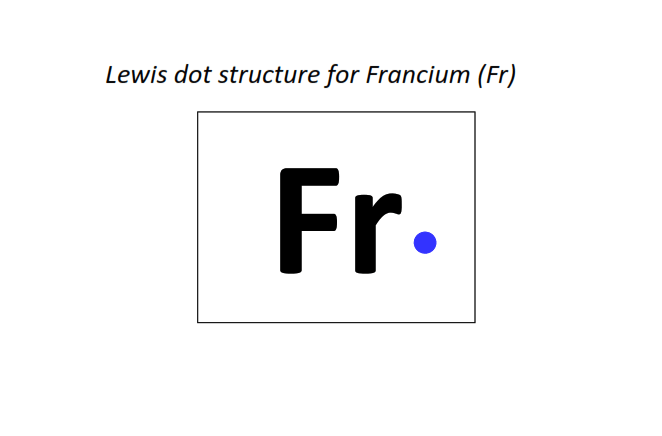
87 | Lewis dot structure of Francium (Fr)[2, 8, 18, 32, 18, 8, 1]
|
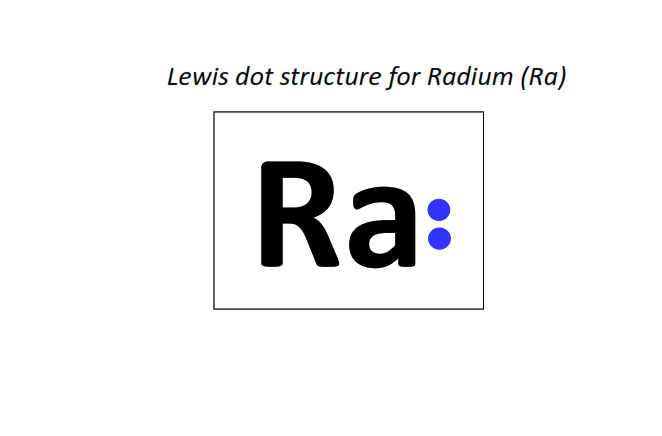
88 | Lewis dot structure of Radium (Ra)[2, 8, 18, 32, 18, 8, 2]
|
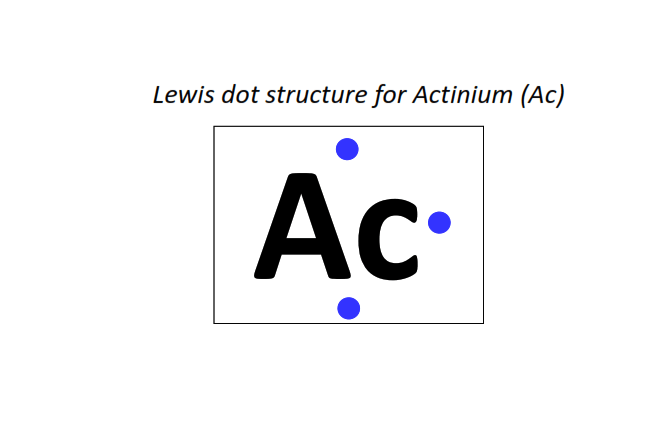
89 | Lewis dot structure of Actinium (Ac)[2, 8, 18, 32, 18, 9, 2]
|
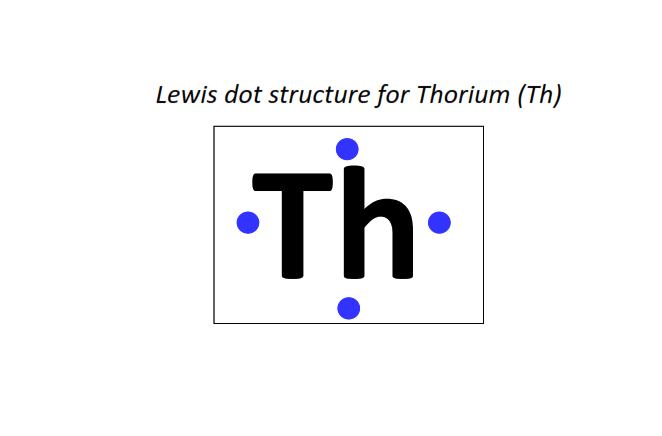
90 | Lewis dot structure of Thorium (Th)[2, 8, 18, 32, 18, 10, 2]
|
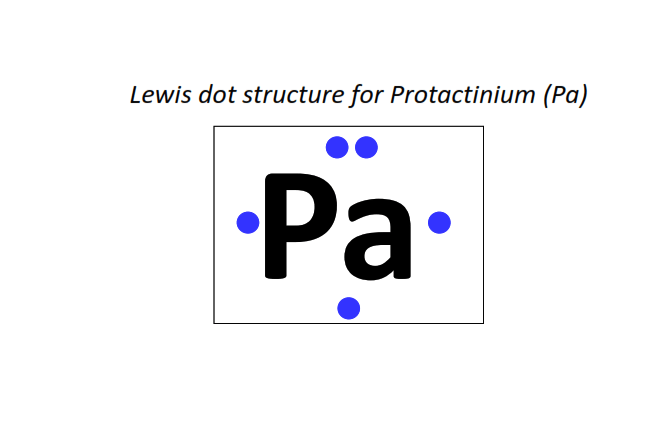
91 | Lewis dot structure of Protactinium (Pa)[2, 8, 18, 32, 20, 9, 2]
|
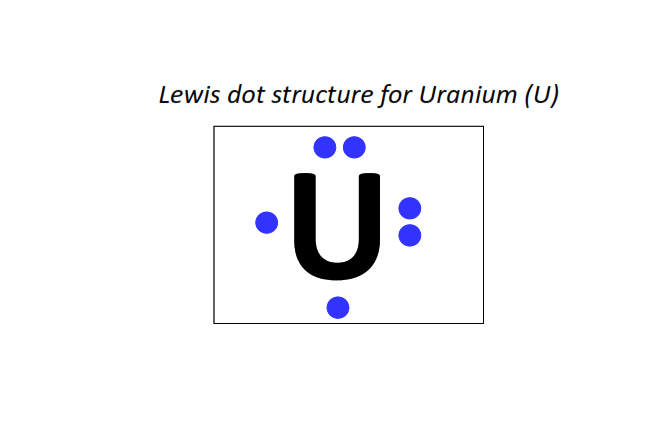
92 | Lewis dot structure of Uranium (U)[2, 8, 18, 32, 21, 9, 2]
|
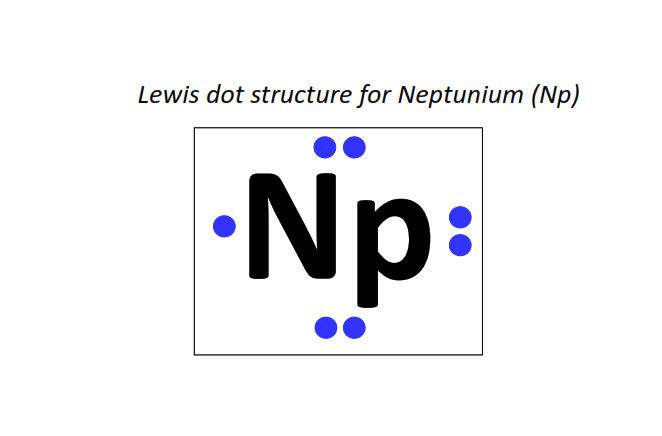
93 | Lewis dot structure of Neptunium (Np)[2, 8, 18, 32, 22, 9, 2]
|
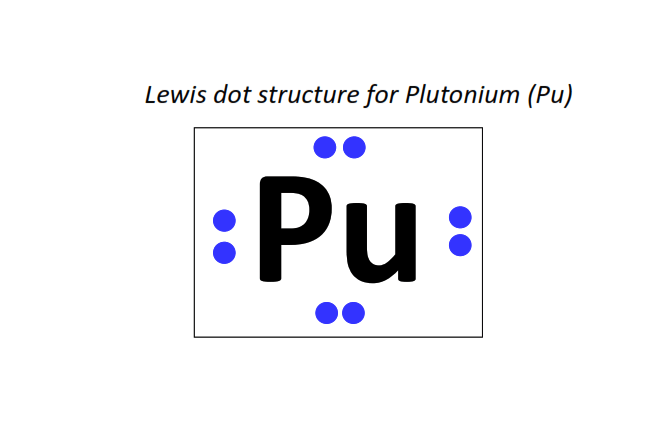
94 | Lewis dot structure of Plutonium (Pu)[2, 8, 18, 32, 24, 8, 2]
|
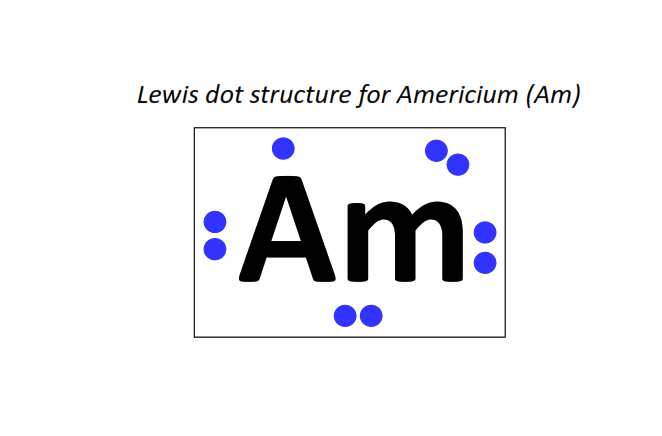
95 | Lewis dot structure of Americium (Am)[2, 8, 18, 32, 25, 8, 2]
|
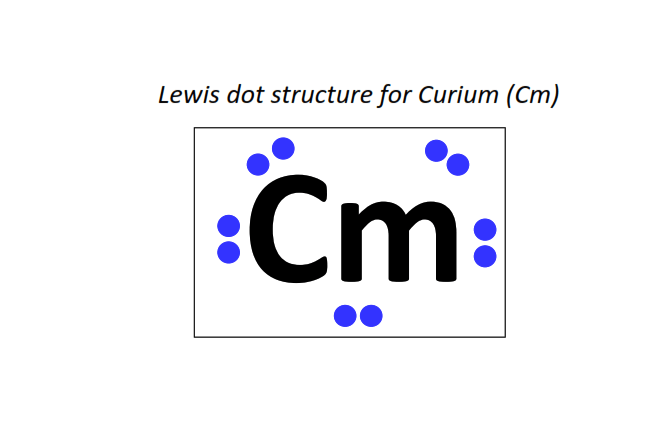
96 | Lewis dot structure of Curium (Cm)[2, 8, 18, 32, 25, 9, 2]
|
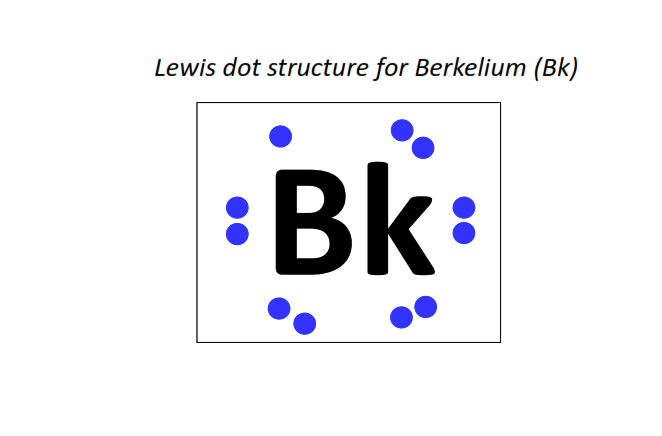
97 | Lewis dot structure of Berkelium (Bk)[2, 8, 18, 32, 27, 8, 2]
|
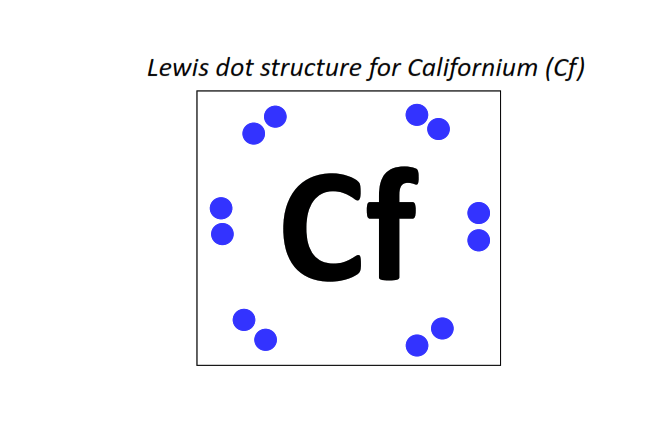
98 | Lewis dot structure of Californium (Cf)[2, 8, 18, 32, 28, 8, 2]
|
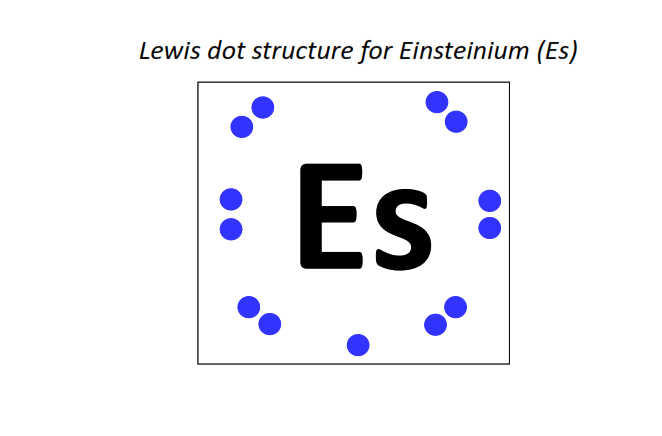
99 | Lewis dot structure of Einsteinium (Es)[2, 8, 18, 32, 29, 8, 2]
|
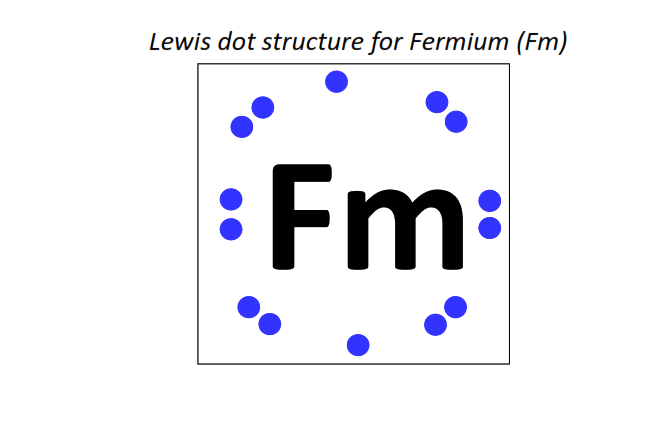
100 | Lewis dot structure of Fermium (Fm)[2, 8, 18, 32, 30, 8, 2]
|
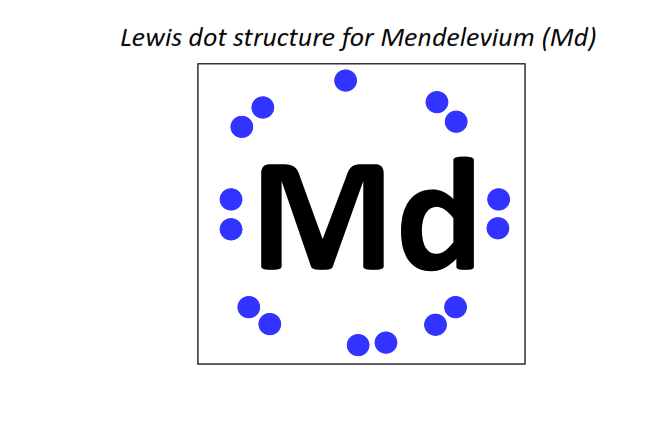
101 | Lewis dot structure of Mendelevium (Md)[2, 8, 18, 32, 31, 8, 2]
|
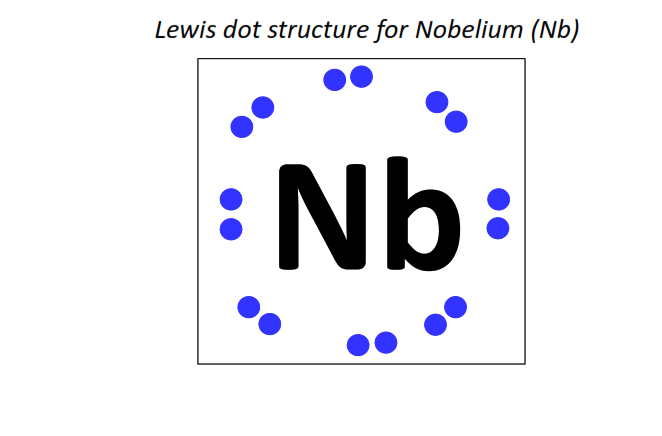
102 | Lewis dot structure of Nobelium (No)[2, 8, 18, 32, 32, 8, 2]
|
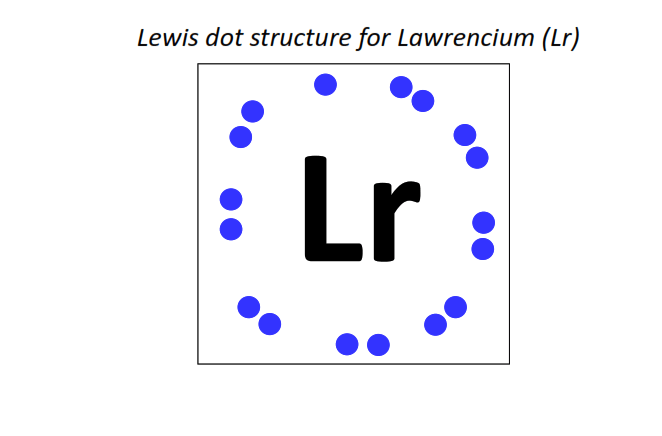
103 | Lewis dot structure of Lawrencium (Lr)[2, 8, 18, 32, 32, 8, 3]
|
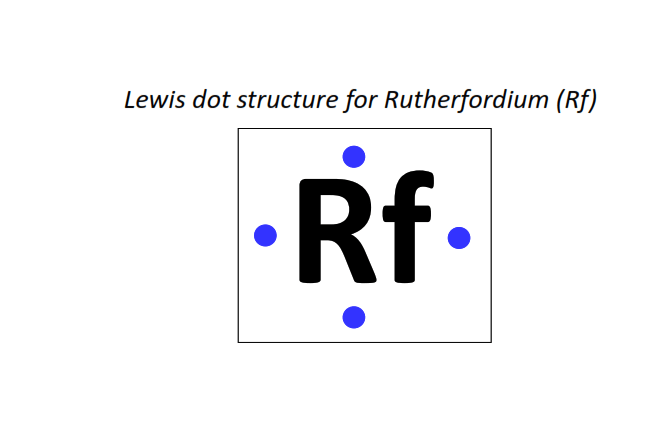
104 | Lewis dot structure of Rutherfordium (Rf)[2, 8, 18, 32, 32, 10, 2]
|
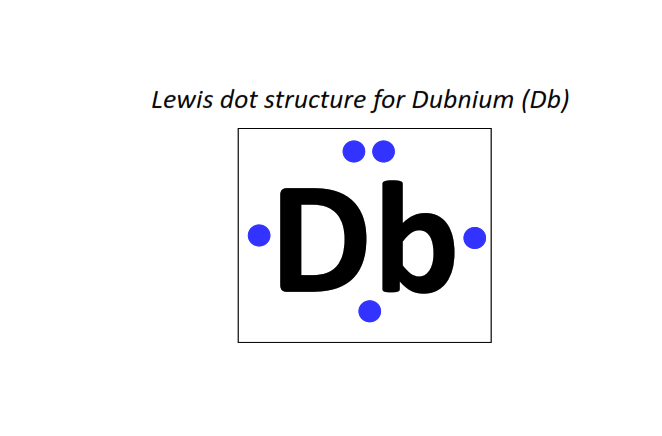
105 | Lewis dot structure of Dubnium (Db)[2, 8, 18, 32, 32, 11, 2]
|
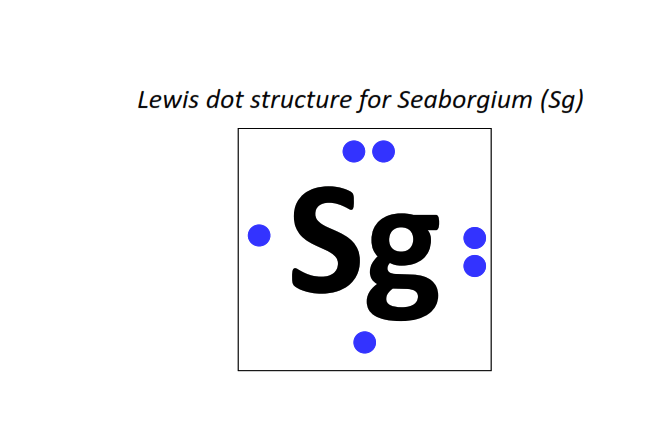
106 | Lewis dot structure of Seaborgium (Sg)[2, 8, 18, 32, 32, 12, 2]
|
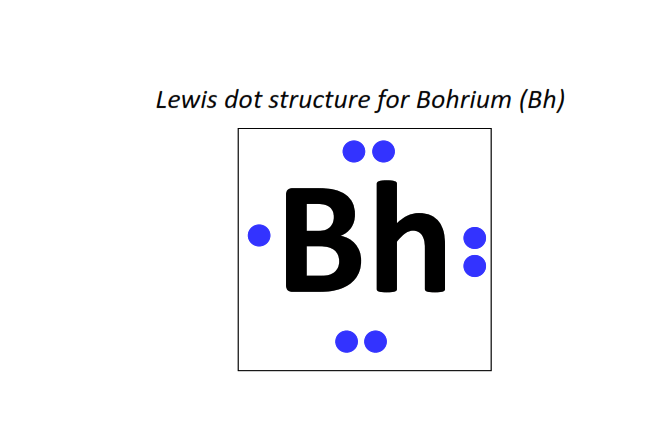
107 | Lewis dot structure of Bohrium (Bh)[2, 8, 18, 32, 32, 13, 2]
|
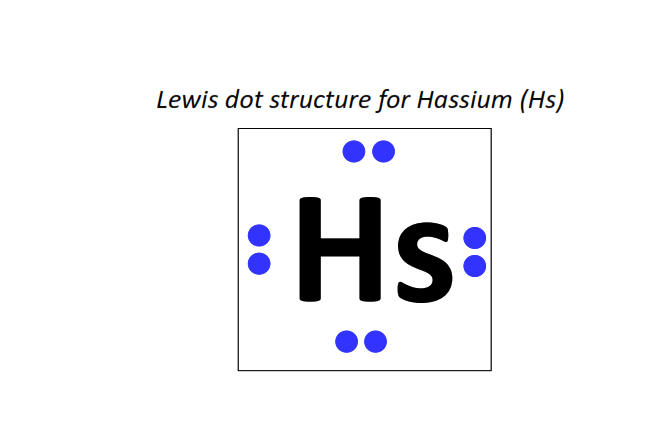
108 | Lewis dot structure of Hassium (Hs)[2, 8, 18, 32, 32, 14, 2]
|
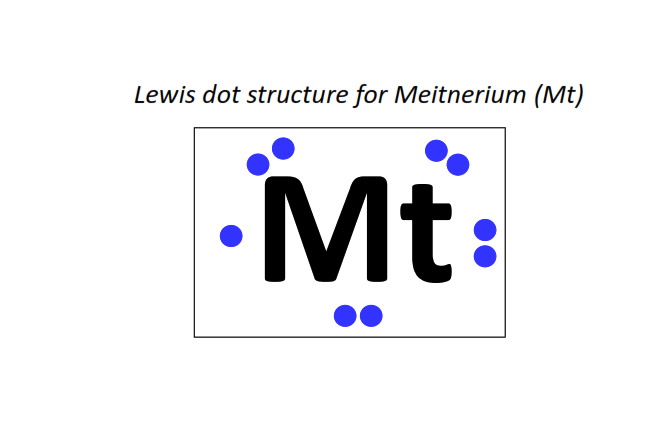
109 | Lewis dot structure of Meitnerium (Mt)[2, 8, 18, 32, 32, 15, 2]
|
110 | Lewis dot structure of Darmstadtium (Ds)[2, 8, 18, 32, 32, 16, 2]
|
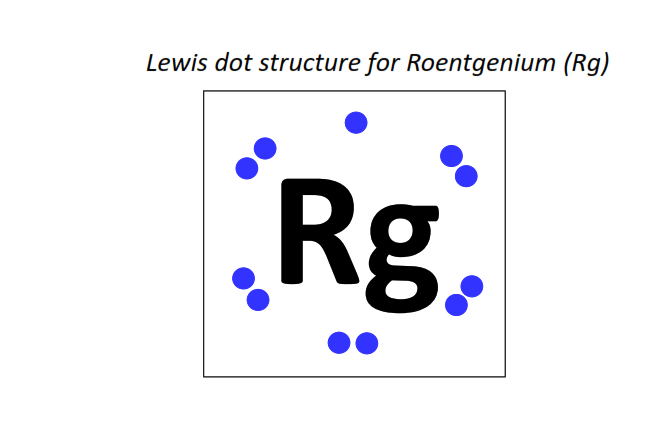
111 | Lewis dot structure of Roentgenium (Rg)[2, 8, 18, 32, 32, 17, 2]
|
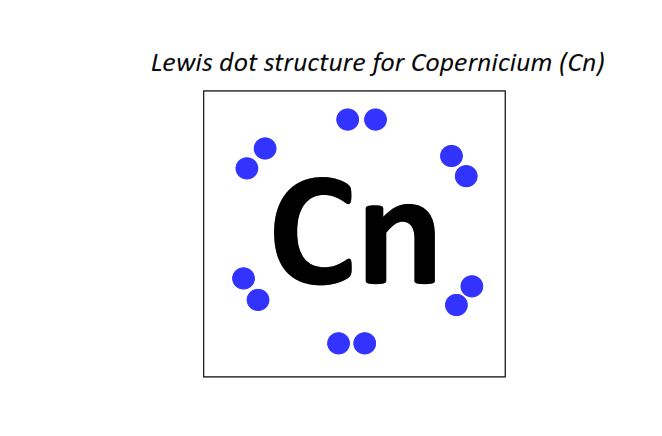
112 | Lewis dot structure of Copernicium (Cn)[2, 8, 18, 32, 32, 18, 2]
|
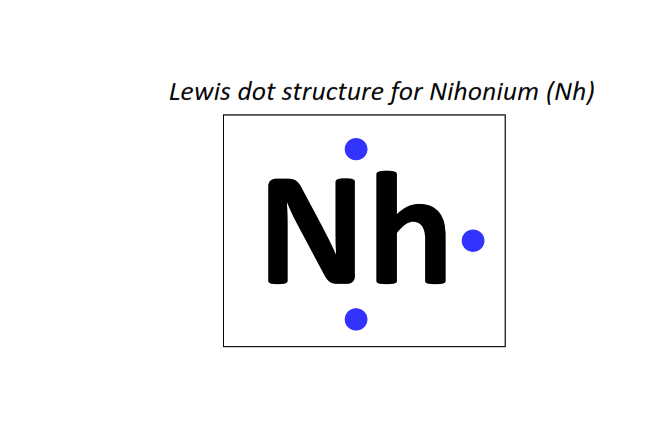
113 | Lewis dot structure of Nihonium (Nh)[2, 8, 18, 32, 32, 18, 3]
|
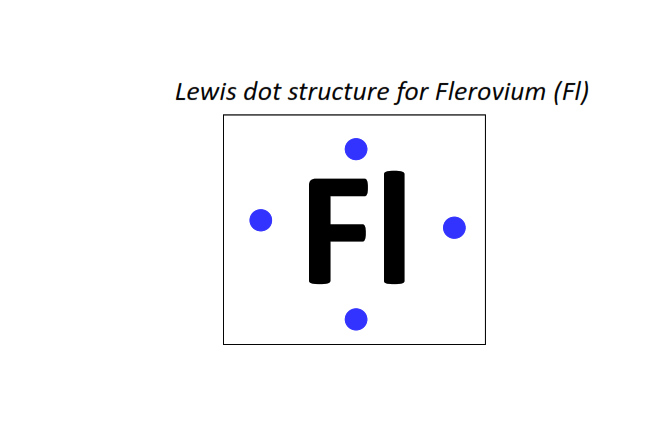
114 | Lewis dot structure of Flerovium (Fl)[2, 8, 18, 32, 32, 18, 4]
|
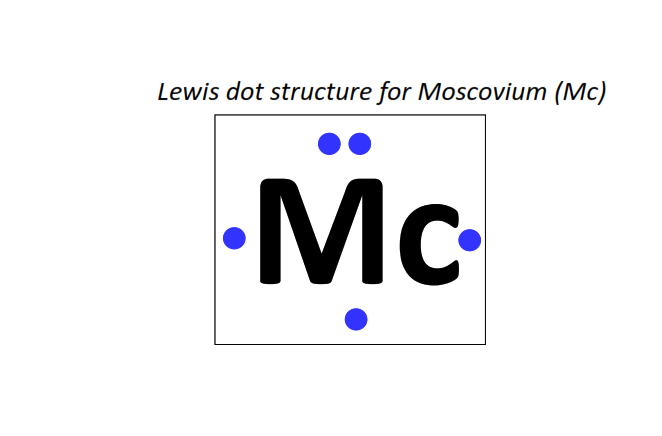
115 | Lewis dot structure of Moscovium (Mc)[2, 8, 18, 32, 32, 18, 5]
|
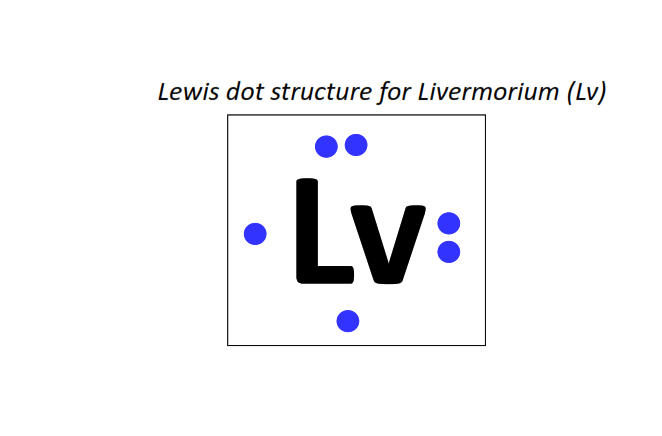
116 | Lewis dot structure of Livermorium (Lv)[2, 8, 18, 32, 32, 18, 6]
|
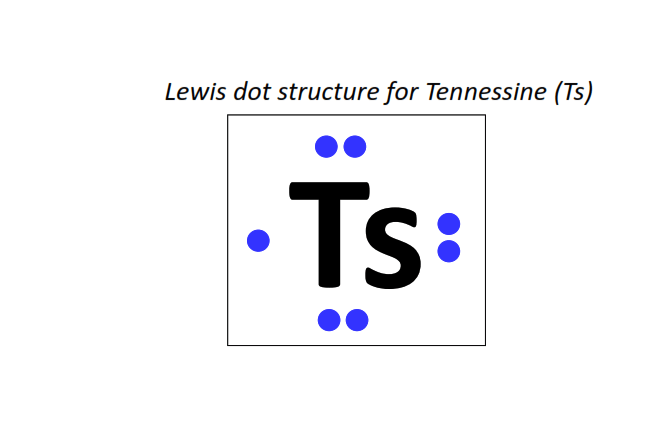
117 | Lewis dot structure of Tennessine (Ts)[2, 8, 18, 32, 32, 18, 7]
|
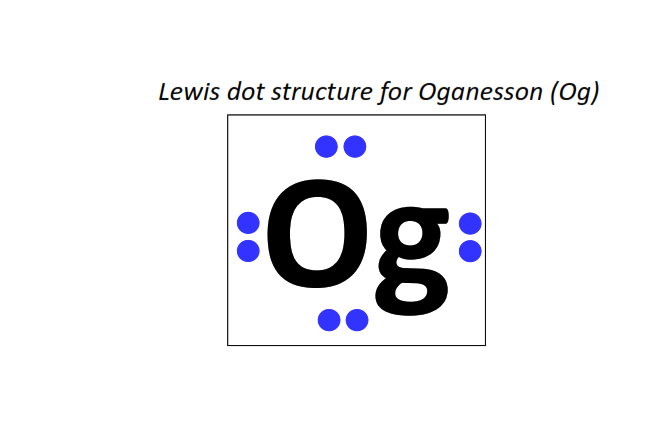
118 | Lewis dot structure of Oganesson (Og)[2, 8, 18, 32, 32 18, 8]
|
Important information:
In this article, we have shown all the valence electrons for d-block elements.
D-block elements are transition metals present in Groups 3-12 of the Periodic Table i.e., from Sc to Cn (except lanthanides and actinides).
These elements can use for bonding their penultimate shell (n-1) d-orbital electrons in addition to those present in the outermost shell (n).
Examples:
⇒The electronic configuration of Scandium (Sc): [Ar] 3d1 4s2
∴ Total number of valence electrons shown in the Lewis structure = electrons in 3d orbitals + electrons in the outermost shell (4s) = 1 + 2 = 3 valence electrons
⇒The electronic configuration of Platinum (Pt): [Xe] 4f14 5d9 6s1
∴ Total number of valence electrons shown in the Lewis structure = electrons in 5d orbitals + electrons in the outermost shell (6s) = 9 + 1 = 10 valence electrons
Similarly, for f-block elements, lanthanides, and actinides, the inner shell (n-2) f-orbital electrons and (n-1) d-orbital electrons are displayed as valence electrons on the Lewis structure along with the outermost shell (n) electrons.
Examples:
⇒The electronic configuration of Cerium (Ce): [Xe] 4f1 5d1 6s2
∴ Total number of valence electrons shown in the Lewis structure = electrons in 4f orbitals + electrons in 5d orbitals + electrons in the outermost shell (6s) = 1 + 1 + 2 = 4 valence electrons
⇒The electronic configuration of Fermium (Fm): [Rn] 5f12 7s2
∴ Total number of valence electrons shown in the Lewis structure = electrons in 5f orbitals + electrons in 6d orbitals + electrons in the outermost shell (7s) = 12 + 0 + 2 = 14 valence electrons
FAQ
What is an Electron dot structure? |
The electron dot structure, often known as the Lewis dot structure, is a graphical representation of the valence electrons of an atom. It uses dots placed around the chemical symbol of an element to indicate its valence electrons. |
How can you draw the Lewis dot structure for each element of the Periodic table? |
To draw the Lewis dot diagram for an atom, follow these basic steps.
|
About the author
Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. The team at Topblogtenz includes experts like experienced researchers, professors, and educators, with the goal of making complex subjects like chemistry accessible and understandable for all. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/