Is HNO2 an acid or base? - Strong or Weak (Nitrous acid)

Nitrous acid is a monoprotic acid with the chemical formula HNO2. It is made up of three elements hydrogen, oxygen, and nitrogen. It only exists in solution and is very unstable in nature. It is used to make diazonium salts from amines.
In this article, we will discuss Is HNO2 an acid or base, Is it strong or weak? its conjugate base, etc.
So, Is HNO2 an acid or base? HNO2 is considered an acid. It releases H+ ions when dissolved in an aqueous solution. And acid is a substance that donates the proton to other compounds or releases H+ ions in a water solution. Hence, HNO2 is acid as it releases H+ ions in a water solution. It has a pH value of 2.67 in 10 mM solution.
| Name of Molecule | Nitrous acid |
| Chemical formula | HNO2 |
| Conjugate base | NO2– |
| Nature | Weak acid |
| Acidity (pKa) | 3.15 |
Why HNO2 act as acid?
An acid is something we know that donates the H+ ion or proton when it is dissolved in a solution.
In the case of HNO2, when dissolved in an aqueous solution, it yields H3O+ and NO2– ions.
⇒ HNO2 + H2O → H3O+ + NO2−
Here in this reaction, HNO2 donates the proton to H2O and formed NO2– conjugate base. And H2O acts as a base because it accepts the proton from HNO2 and forms conjugate acid H3O+.
Theories to check whether HNO2 is an acid or base.
1. Arrhenius’s theory for acid:
This theory said that a substance behaves as an acid when it is ready to give off the hydrogen ion on dissolving in an aqueous solution. And a substance behaves as a base when it releases OH– ion on dissolving in an aqueous solution.
Or a substance is said to be acid when it increases the concentration of H+ ion in solution and a substance is said to be base when it increases the concentration of OH– in solution.
Now check HNO2 nature as per Arrhenius theory–
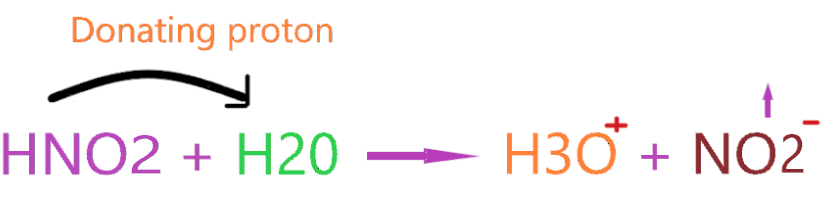
So, the HNO2 molecule, when dissolving in an aqueous solution it dissociates into H+ ions which later forms hydronium ion(H3O+). Also, you can check the number of hydrogen ions before and after in solution to verify Arrhenius acid theory.
In the above reaction, we have only two hydrogens on the left side but after reacting with HNO2, these hydrogens turn into three. Definitely, there is some increment in hydrogen ions.
So, we can proudly say HNO2 is an Arrhenius acid.!
2. Bronsted-Lowry theory for acid:
It states that a compound is an acid when it donates an H+ ion or proton to another compound and itself forms the conjugate base.
Consider the example of HNO2 reacting with H2O.
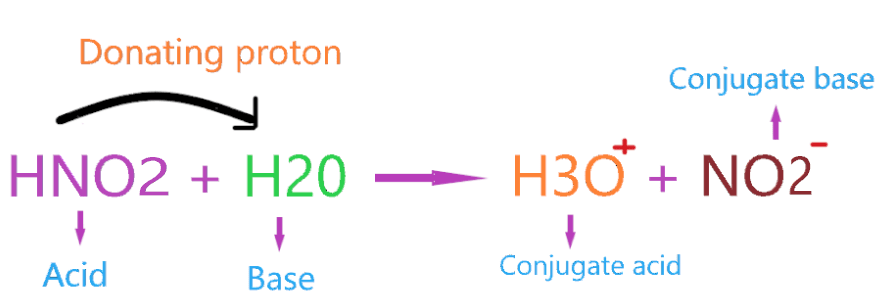
So, HNO2 donates the proton to the H2O molecule and itself forms a conjugate base which is NO2–. Therefore, we can say HNO2 acts as a Bronsted Lowry acid in the above reaction.
Is HNO2 strong acid or weak acid?
The concept of a strong and weak acid is very important to distinguish between the strength of any acid.
A strong acid is referred to as those acids which ionize 100% on dissolving in a solution means all moles of the compound dissociate completely into ions and liberates a large number of H+ in the final solution. Examples of strong acids:- HClO4, HNO3, H2SO4, HI, etc.
Also, Read:-
- Is H2SO4 a strong acid?
- Is HNO3 a strong acid?
- Is HCl a strong acid?
- Is HI a strong acid?
- Is HClO4 a strong acid?
- Is HBr a strong acid?
A weak acid is referred to as those acids which do not ionize 100% on dissolving in a solution means not all moles of the compound dissociate into ions, some molecules are left undissociated in the solution. Examples of weak acids:- H3PO4, CH3COOH, NH4+, HF, H2CO3, etc.
Also, Read:-
- Is CH3COOH a weak acid?
- Is HF a weak acid?
- Is HCN a weak acid?
- Is HCOOH a weak acid?
- Is H3PO4 a weak acid?
- Is H2CO3 a weak acid?
- Is NH4+ a weak acid?
Now, Is HNO2 a strong or weak acid? HNO2 is a weak acid. Since it doesn’t ionize completely to liberate H+ ions on dissolving in an aqueous solution. It means, that when HNO2 is dissolved in an aqueous solution, some parts of it remain undissociated in the solution.
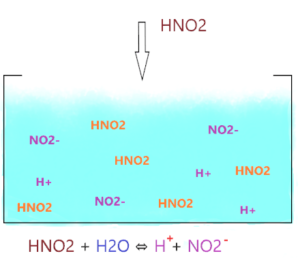
As shown in the figure, when HNO2 is dissolved in water, it dissociates into two ions (NO2– and H+) but the ion(NO2–) is not stable, it wants to combine with H+ and make HNO2 again.
So, not all the HNO2 molecules dissociate, most of them stay together and only a few of them dissociate and liberate H+ ions.
Hence, the number of hydrogen ions in the final solution has a lower amount due to only partial dissociation of HNO2.
Also, the acid dissociation constant value(Ka) for HNO2 is 7.2 × 10-4 which is way lower than recommended value for strong acid.
The acid dissociation constant (Ka) is a quantitative measure of the strength of an acid in solution
⇒ If the value of the dissociation constant of acid is greater than 1 (Ka >> 1), then the nature of the compound is a strong acid.
⇒ If Ka << 1, then the nature of the compound is a weak acid.
Also Read:
List of some important acid and base-

What is the Conjugate base of HNO2?
Conjugate acid is an acid that formed when the parent base compound gains one proton and the conjugate base is a base that formed when the parent acid compound loses one proton.
As per Bronsted-Lowry theory, HNO2 is acid and donates one proton to a water molecule and forms a base(NO2–) known as the conjugate base of an acid(HNO2).

∴ The conjugate base of HNO2 is NO2–.
Is HNO3 stronger acid than HNO2?
Yes, HNO3 is a stronger acid than HNO2, one reason is that HNO2 does not dissociate completely in an aqueous solution to liberate proton or H+ ions whereas HNO3 is nearly 100% ionized in an aqueous solution and completely dissociates into H+ and NO3– ions.
Or check the conjugate base of acids to know the stability of the compound. Here, the HNO3 conjugate base is NO3– which is more stable than the conjugate base of HNO2 which is NO2–.
Because in the case of NO3–, the electron can delocalize over three oxygen but in the case of NO2–, the electron can delocalize over only two oxygen.
So, NO3– electron has a greater volume to stabilize the charge than the NO2–. Therefore, NO3– is more stable than NO2–.
Note: If the conjugate base of A acid is more stable than the conjugate base of B acid, then A is a stronger acid than B acid.
Uses and properties of Nitrous acid
- It is used to make diazonium salts from amines.
- It is nonflammable and soluble in water.
- It has a molar mass of 47.013 g/mol.
- It appears as a pale blue solution.
- It can form water-soluble nitrates and stable esters.
Summary
Here comes the last part! It’s time to rewind this article on HNO2 acid or base with some highlighted or important points.
- Is HNO2 acid or base? HNO2 is an acid. It releases the H+ ion when dissolved in an aqueous solution, making it acidic.
- HNO2 is acting as an Arrhenius acid and Bronsted-Lowry acid.
- The conjugate base of Nitrous acid (HNO2) is NO2–.
- HNO2 is a weaker acid than HNO3.
- HNO2 (Nitrous acid) is a weak acid. Because it does not ionize 100% or only partially dissociates in an aqueous solution.
- The acid dissociation constant value (Ka) for HNO2 is 7.2 × 10-4
About the author
Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. The team at Topblogtenz includes experts like experienced researchers, professors, and educators, with the goal of making complex subjects like chemistry accessible and understandable for all. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/

