Is Hydrogen iodide (HI) an acid or base? Strong or Weak - Conjugate base

Hydrogen iodide belongs to the hydrogen halide family consist of one iodine and one hydrogen with the chemical formula HI. It appears as a colorless gas with a pungent odor. Exposure to this gas can cause strong irritation in the skin, eyes, etc.
In this article, we will discuss Is Hydrogen iodide (HI) is an acid or base? Its nature(Strong or weak).
So, Is HI an acid or base? HI is considered an acid. It releases H+ ions when dissolved in an aqueous solution. And acid is a substance that donates the proton to other compounds or release H+ ions in a water solution. Therefore, HI is acid, since, it releases H+ ions in a water solution.
| Name of Molecule | Hydroiodic acid |
| Chemical formula | HI |
| Molar mass | 127.91 g·mol−1 |
| Nature | Strong acid |
| Conjugate base | I– |
| pH value | 3.01 |
Why HI is an acid?
To Know Why HI is acting as an acid? We have to look into the famous theory given by Arrhenius for the acid compounds.
This theory state that a compound is said to be acid when it produces H+ ions on dissolving in an aqueous solution and forms H3O+ ions when combining with the water molecule.
Now have a look at HI dissociation in an aqueous solution.
⇒ HI → H+ + I–
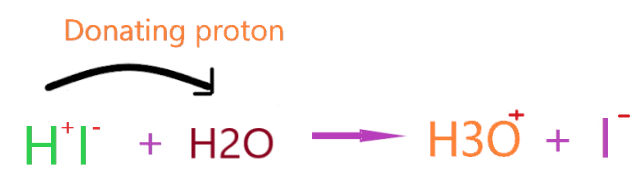
As on dissolving HI in an aqueous solution, it dissociates in two ions H+ and I–. Then, a proton ion(H+) combines with a water molecule and forms H3O+.
⇒ HI(g) + H2O(l) → H3O+(aq) + I−(aq)
Also, Arrhenius sate that an acid is a compound that increases the concentration of hydrogen ion(H+) in solution.
In the case of the HI compound, when dissolved in an aqueous solution it liberates the H+ ions, hence increases the concentration of hydrogen ion in the final solution.
So, the HI compound definitely following all conditions needs to meet for the Arrhenius acid compound. Hence, we can say the HI is an Arrhenius acid compound.
Now we look for another most important acid-base theory that is the Bronsted-Lowry theory.
This theory states a compound is classified as an acid when it donates the proton to other species and itself forms a conjugate base. And a compound is classified as a base when it accepts the proton from other species and itself forms a conjugate acid.
In short as per Bronsted-Lowry theory–
- A Bronsted-Lowry acid is a proton (hydrogen ion) donor.
- A Bronsted-Lowry base is a proton (hydrogen ion) acceptor.
Let’s check whether HI fulfills the requirement for classifying as Bronsted-Lowry acid or not.
Consider the reaction of HI reacting with NH3.
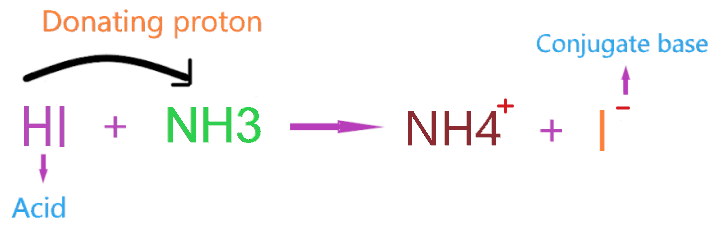
Here, HI reacts with ammonia and donates the proton which is accepted by NH3 and itself forms an I– conjugate base.
Illustration of above reaction (HI with NH3):-
- HI act as Bronsted-Lowry acid as it donates the proton and forms a conjugate base.
- NH3 acts as a Bronsted-Lowry base as it accepts the proton from HI and forms a Conjugate acid.
- Ammonium ion (NH4+) is the conjugate acid of the base NH3.
- Iodine ion(I–) is the conjugate base of the acid HI.
Is Hydroiodic (HI) strong acid or weak acid?
A strong acid is something that completely dissociates or is 100% ionized in a solution. Some examples of strong acid – HCl, HBr, HNO3, H2SO4, etc.
Also, Read:-
- Is H2SO4 a strong acid?
- Is HNO3 a strong acid?
- Is HCl a strong acid?
- Is HBr a strong acid?
- Is HClO4 a strong acid?
Characteristics of strong acid:-
- They are strong electrolytes and have high conductivity.
- Their pH value lies between 1 to 3.
- They dissociate completely and release a large number of H+ ions in a solution.
A weak acid is something that is not able to dissociate completely or partially dissociate in a solution. Some examples of a weak acids – are CH3COOH, H3PO4, HF, NH4+, etc.
Also, Read:-
- Is CH3COOH a weak acid?
- Is HF a weak acid?
- Is HCN a weak acid?
- Is HNO2 a weak acid?
- Is HCOOH a weak acid?
- Is H3PO4 a weak acid?
- Is H2CO3 a weak acid?
- Is NH4+ a weak acid?
Characteristics of Weak acids:-
- They are weak electrolytes and have less conductivity as compared to strong acids.
- Their pH value lies between 3 to 7.
- They ionized partially to produce H+ in a solution.
Now, Is HI a strong or weak acid? HI is a strong acid. Because it readily dissociates in an aqueous solution, which means no undissociated parts of it remain in the solution, all parts completely break off and are ionized in an aqueous solution.
Why HI easily breaks off and dissociate readily in an aqueous solution?
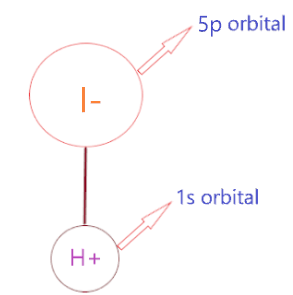
HI can easily break off because the bond strength of the H-I bond is very weak due to the large gap in the orbital size of these ions. As the orbital size for H is 1s and for I atom, it is 5p.
Hence, overlapping of 1s and 5p orbital becomes very small, this causes binding between H-I very weak in nature, hence the covalent bond in H-I atom easily breaks off and makes it readily dissociate in aqueous to liberate H+ ion.
More reasons, why HI acts as strong acid?
As we know, halogen atoms have very high electronegativity and a high energy level, these make a halogen atom size bigger and hydrogen is just opposite to it. So, the attraction between iodine and hydrogen becomes very weak.
Therefore, Hydrogen easily breaks off from HI due to its small size and causes HI to readily dissociates in an aqueous solution and completely splits into two ions (H+ and I–).
Also Read:
What is the conjugate base of HI?
Whenever the acid donates the proton, it converts into the conjugate base and whenever the base accepts the proton, it converts into the conjugate acid.
Conjugate acid is an acid that formed when the base compound gains one proton and the conjugate base is a base that formed when the acid compound loses one proton.
The concept of conjugate acid-base pair.
- A very strong acid always forms a weak conjugate base.
- A very strong base always forms a weak conjugate acid.
- A very weak acid always forms a strong conjugate base.
- A very weak base always forms a strong conjugate acid.
As per Bronsted-Lowry, HI is acid and loses one proton when combines with the water molecule and forms a base known as the conjugate base of an acid(HI).
∴ The conjugate base of HI is I–.
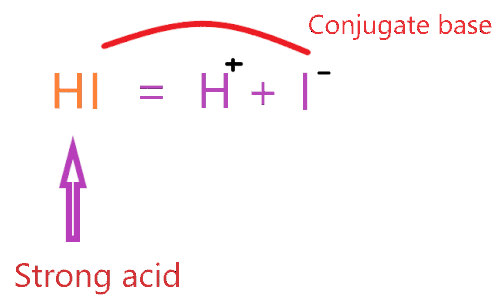
Is HI Lewis acid or base?
When the compound accepts the pair of electrons from another compound classified as Lewis acid and when the compound donates the pair of electrons to another compound classified as Lewis base.
⇒ Lewis acid → electron pair acceptor
⇒ Lewis base → electron pair donator
Now, Is HI Lewis acid or base? Definitely, HI is a lewis acid. Because it accepts one lone pair when combined with a water molecule, forming hydronium ion and I– conjugate base.
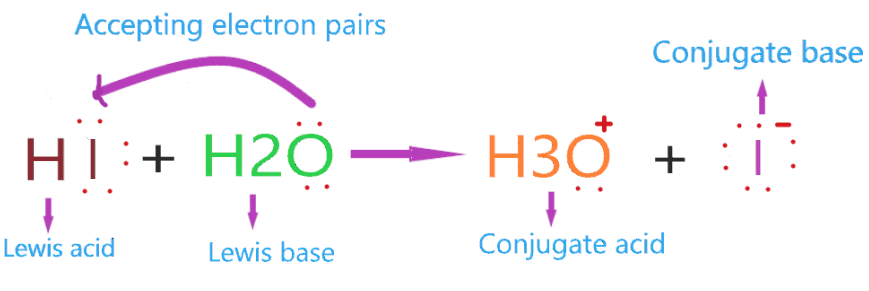
As iodine is more electronegative than hydrogen and has 3 lone pair around it, being more electronegative it attract more electron towards itself, hence a negative charge induces an iodine atom and a positive charge on the hydrogen atom.
The lone pair on the water molecule(H2O) attracted slightly to the hydrogen atom, also more electrons repelled towards the iodine atom due to its negative charge.
Furthermore, a coordinate bond formed between oxygen and hydrogen, and the iodine breaks away as an iodide ion.
So, the whole HI molecule acts as lewis acid as it accepts the pair of electrons from H2O, and in this process, the iodide ion breaks off.
Uses of Hydroiodic acid
- It is used in organic and inorganic synthesis.
- It is used as a reducing agent.
- It is used in Markovnikov and anti-Markovnikov rule.
- It is also used in organic chemistry to convert primary alcohols into alkyl halides.
Properties of Hydroiodic acid
- It has a pKa value of -10 in water.
- It is one of the strongest mineral acids and stronger acids than HCl.
- It has a boiling point of −35.36 °C and a melting point of −50.80 °C.
- It has a terminus molecular shape.
- It has a dipole moment of 0.38 D.
Summary
Hydrogen iodide is an inorganic compound that appears as a colorless gas with a pungent odor having a pH value of 3.01. When hydrogen iodide is dissolved in water, it forms hydroiodic acid. At last, with some important points of this article on Is HI an acid or base? we will finish it.
- HI is a strong acid. It liberates the H+ ions when dissolved in an aqueous solution, therefore, increasing the concentration of hydrogen ions in the solution, making it acidic.
- HI is lewis acid. Because of lone pair electrons accepting ability from another compound.
- The conjugate base of HI is the Iodide ion (I–). It formed after the removal of one proton from Hydroiodic acid (HI).
- Is Hydroiodic (HI) is a strong or weak acid? HI is not only strong acid but it is one of the strongest minerals acids as it completely breaks off when dissolved in an aqueous solution, which means it doesn’t leave any traces of its parts in an aqueous solution, all parts of it completely ionized in solution and increases the concentration of hydrogen ions.
About the author
Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. The team at Topblogtenz includes experts like experienced researchers, professors, and educators, with the goal of making complex subjects like chemistry accessible and understandable for all. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/