Is Hydrogen fluoride (HF) an acid or base? - Strong or Weak

Hydrogen fluoride is a colorless gas that has a strong irritating odor when dissolved in water, it forms hydrofluoric acid that appears as a fuming liquid which can cause painful burns. It has the chemical formula of HF. It is corrosive to metal and tissue.
In this article, we will discuss Is Hydrogen fluoride(HF) is an acid or base? Its nature (Strong or weak), etc.
So, Is HF acid or base? HF is a weak acid, with a pKa of 3.17. When hydrogen fluoride is mixed in an aqueous solution, it gives away one proton (H+) and one (F–). The solution containing one hydrogen (H+) and one fluorine anion (F–) is called hydrofluoric acid.
| Name of Molecule | Hydrogen fluoride |
| Chemical formula | HF |
| Molar mass | 20.006 g/mol |
| Nature | Weak Acid |
| Conjugate base | F– |
| Acidity (pKa) | 3.17 |
Why HF is an acid and not a base?
Do you know? you can easily guess up by looking at the compound whether it is acid or base. Let’s see how.
Most of the acidic compound starts with hydrogen-like H2SO4, HCl, HBr, HNO3, H2CO3, and many more. And most of the basic compounds end with hydroxide groups (OH) like NaOH, KOH, LiOH, etc.
All these acidic compounds, when dissolved in water breaks apart into hydrogen ion and a basic compound breaks apart into OH– ion. Most acid-base theories in chemistry state that acid donates H+ ions and bases donate OH- ions.
In the case of HF, when dissolved in water it furnishes H+ and F– ions in solution. The presence of an H+ ion in an aqueous solution makes the HF nature acidic.
Now check if HF is an acid or base, with the two important acid-base theories we have (a). Arrhenius theory (b). Bronsted-Lowry theory
(a). Arrhenius theory
This theory said that a substance behaves as an acid when it is ready to give off the hydrogen ion on dissolving in an aqueous solution. And a substance behaves as a base when it releases OH– ion on dissolving in an aqueous solution.
Or a substance is said to be acid when it increases the concentration of H+ ion in solution and a substance is said to be base when it increases the concentration of OH– in solution.
Now check HF nature as per Arrhenius’s theory-
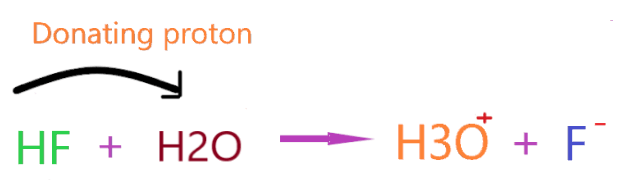
As you see in the above reaction, hydrogen fluoride releases proton on dissolving in an aqueous solution, and, therefore increases the hydrogen ion concentration in the final solution.
Simply, you can check the number of hydrogen ions before and after in solution to verify Arrhenius acid theory.
In the above reaction, we have only two hydrogens on the left side but after reacting with HF, these hydrogens turn into three. Definitely, there is some increment in hydrogen ions.
So, we can proudly say HF is an Arrhenius acid without any doubt!
(b). Bronsted-Lowry theory
Here comes another acid-base theory that states a substance is said to be acid when it donates the proton to other species and makes a conjugate base by losing one proton from itself.
And for the base, it states that a substance is said to be base when it accepts the donated proton and makes a conjugate acid by adding one proton to itself.
Let’s consider the reaction of HF with H2O.
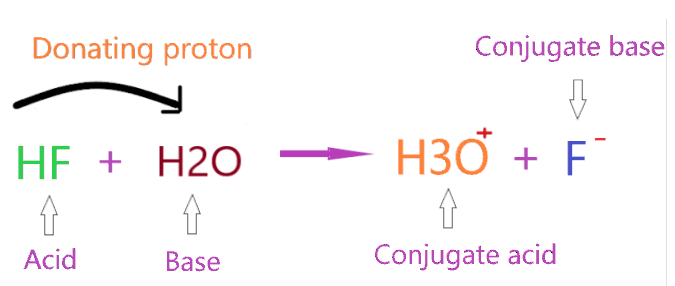
As you see in the above figure, HF donates one proton to the H2O compound and forms a conjugate base(F–) by losing one proton from itself, and H2O accepts this donated proton and makes a conjugate acid (H3O+) by adding one proton to itself.
So, in the above reaction, HF act as a Bronsted-Lowry acid since it donates the proton, and H2O acts as a Bronsted-Lowry base since it accepts the proton.
Is Hydrofluoric (HF) a strong or weak acid?
The concept of a strong and weak acid is very important to distinguish between the strength of any acid.
A strong acid is referred to as those acids which ionize 100% on dissolving in a solution means all moles of the compound dissociate completely into ions and liberates a large number of H+ in the final solution. Examples of strong acids:- HCl, HNO3, H2SO4, HBr, etc.
Also, Read:-
- Is H2SO4 a strong acid?
- Is HNO3 a strong acid?
- Is HCl a strong acid?
- Is HI a strong acid?
- Is HClO4 a strong acid?
- Is HBr a strong acid?
A weak acid is referred to as those acids which do not ionize 100% on dissolving in a solution means not all moles of the compound dissociate into ions, some molecules left undissociated in the solution. Examples of weak acids:- H3PO4, CH3COOH, NH4+, H2CO3, HNO2, etc.
Also, Read:-
- Is CH3COOH a weak acid?
- Is HCN a weak acid?
- Is HNO2 a weak acid?
- Is HCOOH a weak acid?
- Is H3PO4 a weak acid?
- Is H2CO3 a weak acid?
- Is NH4+ a weak acid?
List of some important acid and base-

Now, Is HF a strong or weak acid? HF is a weak acid. Since it doesn’t ionize completely to liberate H+ ions on dissolving in an aqueous solution. It means when HF is dissolved in an aqueous solution, some parts of it remain undissociated in the solution.
Hence, the number of hydrogen ions in the final solution has a lower amount due to only partial dissociation of HF.
Now the question arises, why HF partially dissociates in solution and acquire the weak acid strength?
This is because the bond strength between hydrogen and fluorine is very strong and it requires a large amount of energy to break the bond between H-F in water. Also, fluorine has a very high electronegativity and high affinity toward hydrogen which prevents the ease of releasing H+ in an aqueous solution.
One more thing is that the hydration energy of fluoride ions is lower than the bond energy of H-F, this causes the lower solubility of hydrogen fluoride as well as weak acidity in the aqueous solution.
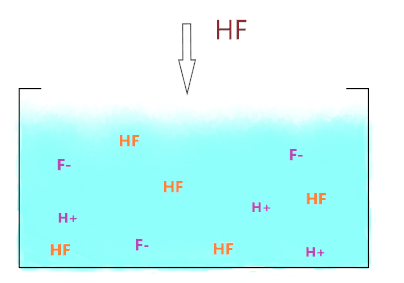 As you see in the above figure when HF molecule is dissolved in an aqueous solution, it starts to split into H+ and F– ions, and due to the weakly acidic nature of HF, it only dissociates partially into ions and some HF molecules don’t break at all and present between the splitting ions in solution.
As you see in the above figure when HF molecule is dissolved in an aqueous solution, it starts to split into H+ and F– ions, and due to the weakly acidic nature of HF, it only dissociates partially into ions and some HF molecules don’t break at all and present between the splitting ions in solution.
Also, the dissociation constant(Ka) value for HF is 6.6 × 10-4, which’s really low for qualifying as a strong acid.
⇒ Ka >> 1 (Strong acid)
⇒ Ka << 1 (Weak acid)
I hope you strongly capture the concept of HF acidic strength.
Also Read:
What is the conjugate base of HF acid?
Conjugate acid is an acid that formed when the parent base compound gains one proton and the conjugate base is a base that formed when the parent acid compound loses one proton.
As per Bronsted-Lowry theory, HF is acid and donates one proton to a water molecule and forms a base(F–) known as the conjugate base of an acid(HF).
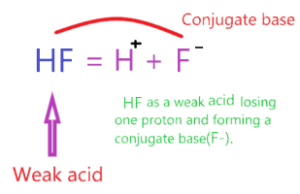
∴ The conjugate base of HF is F–.
Uses of Hydrofluoric acid
- Hydrofluoric acid is used to etch glass and silicon wafers.
- It is used to make most fluorine-containing compounds.
- It is widely used in the petrochemical industry.
- It is also used in pharmaceuticals and polymers.
Properties of Hydrofluoric acid
- It is acidic and highly corrosive in nature.
- It is soluble in water.
- It has a boiling point of 19.5 °C and a melting point of −83.6 °C.
- It has an irritating odor.
Summary
Hydrogen fluoride is a highly dangerous gas that can be converted into hydrofluoric acid by dissolving in an aqueous solution. We covered the important detail of the acidity nature of HF in this article.
- The pKa value of HF is 3.17.
- Is Hydrofluoric (HF) an acid or base? HF is an acid. It has a proton to lose when dissolved in an aqueous solution.
- HF is acting as Arrhenius acid as well as Bronsted-Lowry acid.
- The conjugate base of Hydrofluoric acid (HF) is F–.
- However, HF is a weak acid, due to only partial dissociation of its ions in an aqueous solution, also the dissociation constant(Ka) for HF is 6.6 × 10-4, which is considered far low for the strong acid.
About the author
Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. The team at Topblogtenz includes experts like experienced researchers, professors, and educators, with the goal of making complex subjects like chemistry accessible and understandable for all. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/