Is Bicarbonate (HCO3-) an acid or base? Conjugate acid vs Conjugate base

Bicarbonate is a polyatomic ion made up of one hydrogen, one carbon, and three oxygen with one negative formal charge having a chemical formula HCO3–. It is also known as hydrogen carbonate. It is mainly used in pH buffering systems. They are mostly soluble in water at standard temperature and pressure. Bicarbonate is generated when one proton is removed from carbonic acid.
In this article, we will discuss Is Hydrogen carbonate(HCO3–) an acid or base? It’s conjugate acid as well as conjugate base, etc.
So, Is HCO3– an acid or base? Bicarbonate (HCO3–) is amphoteric, which means it can act as an acid as well as base, depending on what it is reacting with. It is one of the main components used in a pH buffering system due to its amphoteric(acid or base) nature.
Let’s discuss in more detail how can HCO3– can act as an acid as well as a base?
| Name of Molecule | Bicarbonate |
| Chemical formula | HCO3– |
| Molar mass | 61.0168 g mol−1 |
| Nature | Amphoteric |
| Conjugate acid | H2CO3 |
| Conjugate base | CO32- |
| Acidity(pKa) | 10.3 |
| Basicity(pKb) | 7.7 |
Why HCO3- act as acid?
To get a better perspective of the acidic nature of HCO3–. We first look at what the term “Acid” means.
Acid is generally a compound that has a pH value of less than 7 and contains one or more replaceable hydrogen ions. When acidic compounds are dissolved in an aqueous solution, they will release H+ ions.
The more the number of hydrogen ions liberated in an aqueous solution, the higher the strength of that acidic compound.
So, now Why does HCO3– act as an acid? HCO3– can act acid only in one condition when the reacting compound is more basic than it e.g. OH–. This is because a stronger base has more ability to “accept the proton”.
Hence, HCO3– has to donate the proton when reacting with a strong base, and anything that donates the proton is considered acid in nature.
HCO3– act as acid when reacting compound have smaller Ka than it.
⇒ Ka for HCO3– = 4.7 × 10-11
⇒ Ka for OH– = > 10-14 Approx
So, reacting compound(OH–) have smaller Ka, hence, here, HCO3– will act as acid.
Therefore, when HCO3– reacted with a stronger base like OH-, it left with no choice and have to release proton.
A general rule to distinguish between base and acid compounds:-
- If A compound release more hydrogen ions(H+) than B, then compound A is more acidic than B.
- If A contains releases more hydroxide ions(OH–) than B, then compound A is more basic than B.
So, when HCO3– reacts with a compound having OH– ion, then HCO3– will act as an acid and therefore, have to donate a proton. And anything that donates the proton is considered as acid as per Bronsted-Lowry theory.
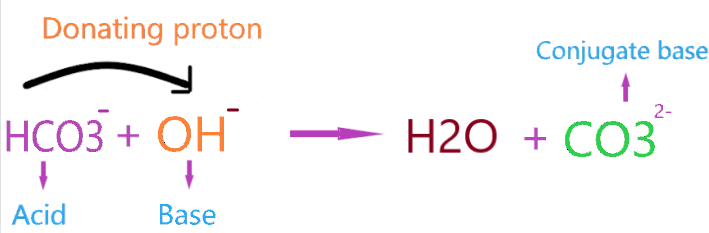
As we see in the above reaction, HCO3– reacts with a base(OH–), therefore donating one proton to a hydroxide ion and making a conjugate base(CO32-).
Bronsted-Lowry’s theory for acid said that “the acid is the substance that donates the proton to reacting species and itself makes a conjugate base.”
As we see in the above figure, HCO3– is donating the proton to OH– and making a conjugate base(CO32-).
Therefore, we can say HCO3– acts as Bronsted-Lowry acid if reacting compound is a stronger base than it.
Also check:-
Why HCO3- act as base?
A base is defined as a proton acceptor or electron-pair donor. A stronger base liberates more OH– when dissolved in an aqueous solution and has a pH value of more than 7.
So, Why does HCO3– act as a base? HCO3– acts as a base when reacting with the compound that is more acidic than it, i.e. HCl. This is because a stronger acid has more ability to “donate the proton”.
Hence, HCO3– has to accept the proton when reacting with a strong acid, and anything that accepts the proton is considered base in nature.
HCO3– act as base when reacting compound have larger Ka than it.
⇒ Ka for HCO3– = 4.7 × 10-11
⇒ Ka for HCl = 1.3 × 106
So, reacting compound(HCl) have larger Ka, hence, here, HCO3– will act as base.
Therefore, when HCO3– reacts with stronger acid like HCl, it is left with no choice and has to accept the proton from HCl. And we know anything that accepts the proton act as a base.
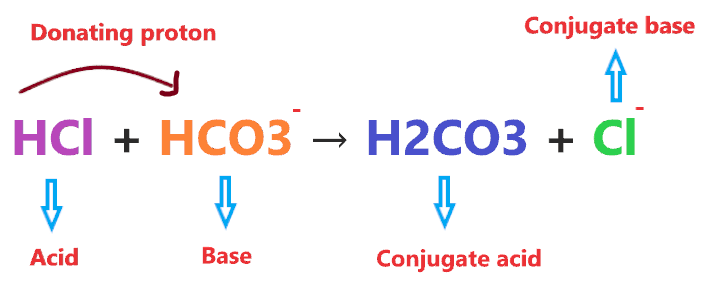
Illustration of above reaction:-
- HCl donates a proton to HCO3– and formed a conjugate base(Cl–). Hence, it is a Bronsted-Lowry acid.
- Bicarbonate(HCO3–) accepts the proton from HCl and forms conjugate acid(H2CO3). Hence, it is a Bronsted-Lowry base.
As per Bronsted-Lowry base theory, a compound is said to base when it accepts the proton from reacting species and forms a conjugate acid by adding one proton to itself.
Therefore, we can say HCO3– can act as a Bronsted-Lowry base if react with strong acid.
Also read:-
What is the conjugate base and conjugate acid of HCO3-?
To know what is the conjugate acid-base of HCO3–, first, we have to through the concept of conjugate acid-base pairs.
The concept of conjugate acid-base pair.
- A very strong acid always forms a weak conjugate base.
- A very strong base always forms a weak conjugate acid.
- A very weak acid always forms a strong conjugate base.
- A very weak base always forms a strong conjugate acid.
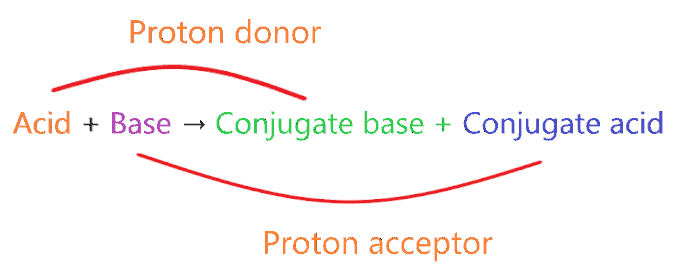
Conjugate acid:-
A conjugate acid is a species formed by gaining one electron by base compound or you can say when the proton is added to the parent base then the compound is formed which is called conjugate acid. Example of conjugate acid-
⇒ NH3 + H+ → NH4+
Here in this reaction, NH3 is a weak base that accepts the proton when dissolved in an aqueous solution and forms conjugate acid(NH4+).
So, NH4+ is the strong conjugate acid of NH3.
Conjugate base:-
A conjugate base is a species formed by losing one electron by an acid compound or you can say when the proton is removed from the parent acid then the compound is formed which is called the conjugate base. Examples of conjugate base-
⇒ HCl → H+ + Cl–
Here, in this reaction, HCl is a strong acid that donates the proton when dissolved in an aqueous solution and formed a conjugate base(Cl–).
So, Cl- is the weak conjugate base of HCl.
⇒ H2SO4 → H+ + HSO4–
Here, in this reaction, H2SO4 is a strong acid that donates the proton when dissolved in an aqueous solution and formed a conjugate base(HSO4–).
So, HSO4– is a weak conjugate base of H2SO4.
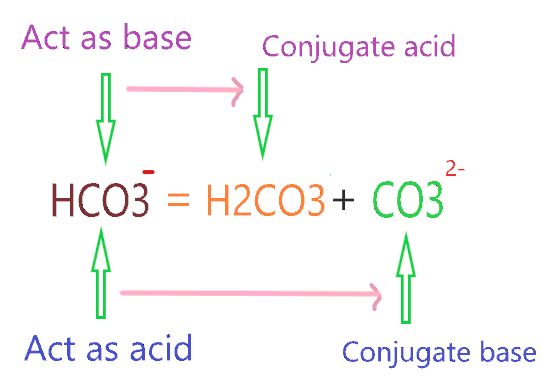
Now, what is the conjugate acid and base of HCO3–? When HCO3– acts as an acid it will make a conjugate base and when it acts as the base it will make conjugate acid.
⇒ When HCO3– react with stronger acid than it such as HCl, it accepts the one proton from HCl and formed a conjugate acid(H2CO3).
⇒ When HCO3– reacts with a stronger base than it such as OH–, it donates the one proton to OH– and formed a conjugate base(CO32-).
Also Read:– Is H2CO3 an acid or base?
Uses of Bicarbonate ion
- Bicarbonate is used to maintain acid-base (pH buffering system) in the human body to counteract the activity of highly acidic digestive juices.
- The salts of bicarbonate ions are used as leavening agents.
- It is one of the important sinks in the carbon cycle.
Summary
“Bicarbonate is an intermediate form in the deprotonation of carbonic acid.” Its acidity value is 10.3 and its basicity is 7.7. The nature of HCO3– is amphoteric means it either acts as an acid or as a base depending on some conditions.
- The conjugate acid of HCO3– is Carbonic acid (H2CO3).
- The conjugate base of HCO3– is a Carbonate ion (CO32-).
- Is bicarbonate (HCO3–) acid or base? HCO3– is both an acid and base, depending on the strength of reacting compound.
- In presence of a strong base like OH–, HCO3– acts as an acid.
- In presence of a strong acid like HCl, HCO3– acts as a base.
About the author
Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. The team at Topblogtenz includes experts like experienced researchers, professors, and educators, with the goal of making complex subjects like chemistry accessible and understandable for all. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/

