Is H2SO4 an acid or base? Strong vs Weak - Conjugate base

Sulfuric acid is colorless and odorless made up of the elements hydrogen, oxygen, and sulfur with the chemical formula H2SO4. This acid is also known as oil of vitriol and it is soluble in water at any concentration. It is corrosive to metal and tissue.
In this article, we will discuss Is H2SO4 an acid or base? Is it strong or weak? Conjugate acid-base of H2SO4, etc.
So, Is H2SO4 an acid or base? H2SO4 is a strong acid. In an aqueous solution, it dissociates into 2H+ and SO42-, So, it has 2H+ ions that are replaceable that correspondingly showing its acidic nature, although, H2SO4 is one of the strongest acids, but, in the presence of superacids, it can act as a base also.
The pH value of H2SO4 is around 1 to 3 but it depends on its concentration.
When reacted with other elements or compounds it can release a huge amount of energy due to its high reactivity properties.
| Name of Molecule | Sulfuric acid |
| Chemical formula | H2SO4 |
| pH value | 1 to 3 |
| Conjugate base | HSO4– |
| Nature | Strong acid |
| Acidity (pKa) | -2.8, 1.99 |
| Solubility in water | miscible |
Why H2SO4 acts as an acid?
H2SO4 acts as an acid because it dissociates into two ions on dissolving in an aqueous solution and liberates proton or H+ ion.
As per various acid theories, an acid is something that releases protons or H+ ions when dissolved in an aqueous solution. Since H2SO4 releases H+ ions in an aqueous solution, it acts as an acid.
⇒ H2SO4 → 2H+ + SO4 – –
We have two important theories to check whether H2SO4 acid or not. (a). Arrhenius theory (b). Bronsted-Lowry theory
1. Arrhenius’s theory for acid:
According to Arrhenius’s concept of acid, an acid is a substance that increases the concentration of H+ ions in an aqueous solution. In the case of H2SO4, it dissociates into two ions(2H+ and SO4– -) on dissolving in an aqueous solution. Hence, it acts as an Arrhenius acid.
2. Bronsted-Lowry theory for acid:
This theory state that acid is the substance that donates the proton to other species and itself forms the conjugate base. So, in the case of H2SO4, when it reacts with H2O then it donates the proton to H2O and itself forms HSO4– conjugate base.
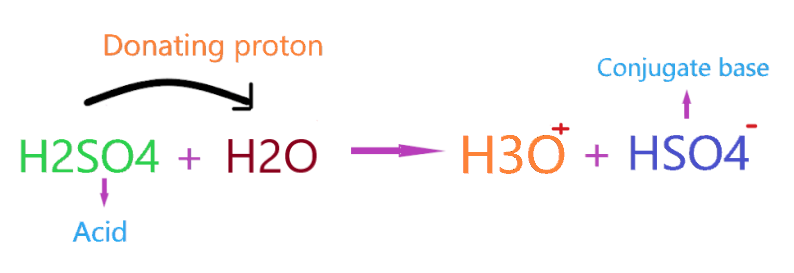
As you see in the above reaction, H2SO4 donates the one H+ ion to H2O and itself forms the conjugate base HSO4–. Hence, as per Bronsted-Lowry theory, H2SO4 acts as an acid whereas H2O acts as a Bronsted-Lowry base.
Is Sulfuric acid (H2SO4) strong or weak?
For knowing whether H2SO4 is a strong or weak acid, first, we have to understand what is a strong acid and weak acid.
A strong acid is a compound that is ionized 100% in an aqueous solution and produced a high concentration of H+ ion in the solution. More is the concentration of H+ ion in an aqueous solution, and less is the pH value of that compound.
Examples of strong acids – are HClO4, HBr, HNO3, HCl, HI, etc.
Also Read:-
- Is HBr a strong acid?
- Is HNO3 a strong acid?
- Is HCl a strong acid?
- Is HI a strong acid?
- Is HClO4 a strong acid?
And weak acid is a compound that partially dissociates to yield H+ ions which means some fraction of the compound is ionized but most of it remains undissociated in the solution.
Almost all organic acids like HNO2, HSO3, etc. are weak acids and their pH value lies between 5 to 7.
Some more examples of weak acids:- Hydrocyanic acid(HCN), Acetic acid(CH3COOH), Ammonium ion (NH4+), and Phosphoric acid(H3PO4), HNO2, HF, HCOOH, H2CO3, etc.
Also Read:
- Is CH3COOH a weak acid?
- Is HF a weak acid?
- Is HCN a weak acid?
- Is HNO2 a weak acid?
- Is HCOOH a weak acid?
- Is H3PO4 a weak acid?
- Is H2CO3 a weak acid?
- Is NH4+ a weak acid?
Now, Is H2SO4 strong acid or weak acid? Sulfuric acid (H2SO4) is a strong acid. Because when it is dissolved in an aqueous solution, it completely dissociates into H+ and HSO4– ions in the solution. There is no undissociated H2SO4 present in the solution, it completely breaks off into the ions.
Sulfuric acid is also called the “king of chemicals or acid” because of its strong acid and more reactive nature.
The presence of more H+ ions in the solution defines sulfuric acid as a good conductor of electricity and a strong electrolyte.
To further understand Why H2SO4 is a strong acid? we have to focus on two reactions that occur after dissolving sulfuric acid in the aqueous solution.
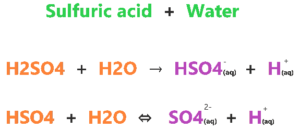
In the first reaction, as you see, a single arrow is used that shows only a forward reaction will occur because H2SO4 completely dissociates into H+ and HSO4–. And this reaction is irreversible means, it strongly favors the right side.
HSO4– is a weak acid and stable conjugate base of H2SO4, hence, it will not reform with H+ to form H2SO4 again in an aqueous solution.
In the second reaction, HSO4– is a weak acid that also partially dissociates and releases one proton and forms SO42-. The double arrow in the second reaction shows that both backward and forward reactions will occur at equilibrium.
(H+ and SO42-) will reform and produce the HSO4– again in solution but there is no effect on the first reaction. Hence, this is why H2SO4 is a strong acid in nature as it fully dissociates into ions in one shot.
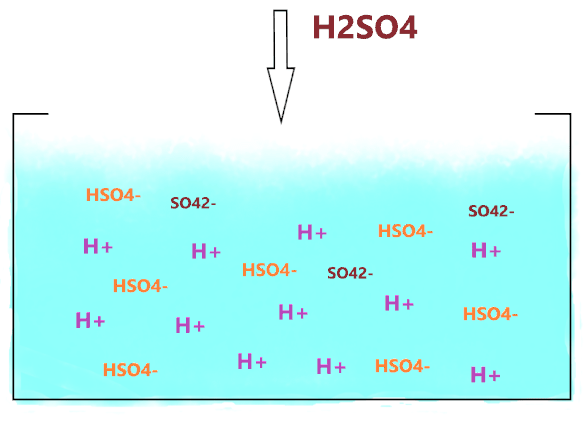
So, as you see, in the above figure, when H2SO4 is dissolved in an aqueous solution, it completely dissociates into two ions(H+ and HSO4–), hence, there is no undissociated H2SO4 molecule present in its aqueous solution that correspondingly shows its very strong acid nature.
Here’s the list of some common strong and weak acids.

Also Read:
Is H2SO4 also act as base?
Yes, sometimes H2SO4 can also act as a base if it reacts with superacids. “A superacid is an acid with an acidity greater than that of 100% pure sulfuric acid” For example – CF3SO3H and HSO3F, both are thousand times stronger than sulfuric acid.
A compound is said to be base when it accepts the proton or donates the OH– in an aqueous solution.
So, when sulfuric acid reacts with superacids then it will accept the proton from super acid, hence, acting as a base.
Super acid always dominates the reacting compound and strongly liberates the H+ ions, so reacting compound has no choice left and has to accept the proton of superacid.
H2SO4 acts as a base:
- When the surrounding compound is superacid.
- When the surrounding compound is more reactive in nature than H2SO4.
Is H2SO4 lewis acid or base?
Lewis acid is a compound that accepts the pair of electrons and lewis base which donates the pair of electrons to other compounds.
So, Is H2SO4 lewis acid or base? H2SO4 is a lewis acid. Because it has an H+ ion that can accept the electron pairs from a compound donor. Almost all simple cation is lewis acid, for example- H+, Na+, Mg+2, etc. Some exceptional cases NH4+, PH4+, etc. are not lewis acid.
When H2SO4 is dissolved in an aqueous solution it gives H+ ions that can accept the pair of electrons from surrounding atoms to complete the deficiency of electrons.
To be a Lewis acid, the atom needs an empty orbital in its valence shell. H+ ion has empty orbital as one electron of it stripped from orbital due to positive charge.
⇒ Lewis acid → electron pair acceptor
⇒ Lewis base → electron pair donator
Note: Lewis acid is a superset of Bronsted-Lowry acids which means every compound that falls into the category of Bronsted-Lowry acid is Lewis acid.
“A Lewis acid is a molecule that accepts pair of electrons, a Bronsted acid is a molecule that donates a proton. The acid will have both of these characteristics.”
What is the Conjugate base of H2SO4
Conjugate acid is always formed by base and conjugate base is formed by acid. When the H+ ion is removed from acid then the compound is formed called the conjugate base of that acid. And when the H+ is added to the base then the compound is formed called conjugate acid of that base.
The concept of conjugate acid-base pair.
- A very strong acid always forms a weak conjugate base.
- A very strong base always forms a weak conjugate acid.
- A very weak acid always forms a strong conjugate base.
- A very weak base always forms a strong conjugate acid.
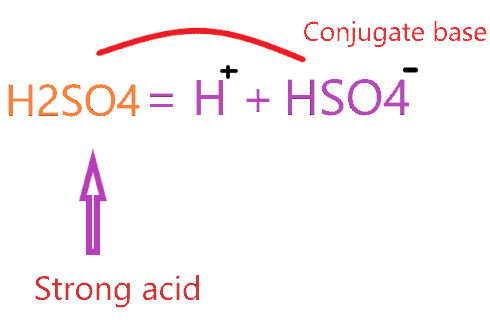
So, H2SO4 is a very strong acid that makes a weak conjugate base according to the concept of conjugate acid-base pair.
The conjugate base of H2SO4 is HSO4–.
Is H2SO4 stronger acid than H2SO3?
Always remember “Greater is the oxidation number of the central atom more is the acidic nature of compound”. It is because greater oxidation numbers attract more electrons towards itself, therefore, expanding the acidity which helps in stabilizing the conjugate base of the compound.
H2SO4 central atom(sulfur) has a +7 oxidation number whereas the H2SO3 central atom(sulfur) has a +4 oxidation number. Clearly, sulfuric acid has high oxidation number than sulfurous acid.
So, H2SO4 central atom pulls out more electron density from the acidic proton. Hence, it is a relatively stronger acid than H2SO3.
Also, H2SO4is diprotic acid that produces two H+ per molecule when dissociating in water or in an aqueous solution.
Uses of Sulfuric acid
- It majorly uses for the production of fertilizer ammonium sulfate and superphosphate.
- At high concentration, it is used as an ingredient in acidic drain cleaners that further used for removing the tissue paper, grease, etc.
- Sulfuric acid acts as the electrolyte in lead-acid batteries.
- It is used for making explosive material.
- In the inorganic industry, H2SO4 is used to produce hydrochloric or hydrofluoric acid.
- It is used in the production of dyes and pharmaceuticals.
- In laboratories, sulfuric is used to test the nature of various chemicals.
- It is also used as a dehydrating agent.
- Sulfuric acid is used in petroleum refining to wash impurities of gasoline and other refinery products.
- It is also used for cleaning iron and steel.
Conclusion
Sulfuric acid is very dangerous and king of chemicals due to its highly reactive nature and low pH value. It is one of the important chemicals to be used in industrial areas. It is soluble in water at high concentrations and corrosive to metal and tissue. Some important points of this article on whether H2SO4 is an acid or base?
- H2SO4 is highly reactive and a strong acid in nature with a pH value between 1 to 3.
- Is H2SO4 an acid or base? H2SO4 acts as an acid. It gives H+ ions on dissolving in an aqueous solution.
- Sulfuric acid (H2SO4) is lewis acid. Because it has an H+ ion that can accept the electron pairs from a compound donor.
- Sulfuric acid (H2SO4) is a strong acid. Because it is completely ionized or dissociated in an aqueous solution and for every 1 mole, it gives two H+ ions and one SO42-.
- The conjugate base of H2SO4 is HSO4-.
- Sometimes H2SO4 can also act as a base when it reacts with superacids.
About the author
Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. The team at Topblogtenz includes experts like experienced researchers, professors, and educators, with the goal of making complex subjects like chemistry accessible and understandable for all. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/


Wow, this post is fantastic! I really appreciate the way you explained H2SO4 as a strong acid. Your explanation was easy to understand.