How to determine if compound is Ionic or Covalent?
Atoms of the same or different elements combine to form substances called compounds through chemical bonding. These compounds formed could be ionic or covalent. How do we know which type of compound is it? How do their properties get affected by their ionic or covalent nature?
We will answer in this article both the questions by discussing the many interesting facts and concepts related to ionic and covalent substances.
In this article, we will discuss the following things-
- How to tell if the compound is ionic or covalent?
- How to know if a compound is ionic or covalent by its formula?
- How to determine if a bond is ionic or covalent?
- How to know if a covalent bond is polar or non-polar?
- How to tell if a substance is ionic or covalent from its properties?
- How to determine if a bond is ionic or covalent using electronegativity?
- How to know if the compound is ionic or covalent according to Fajan’s rule?
|
The best way to tell if the compound is ionic or covalent –
|
How to tell if a compound is ionic or covalent?
This question is difficult to answer in a straightforward manner because there are many interlinked concepts that will get unfolded as you read further.
But as a general matter of fact,
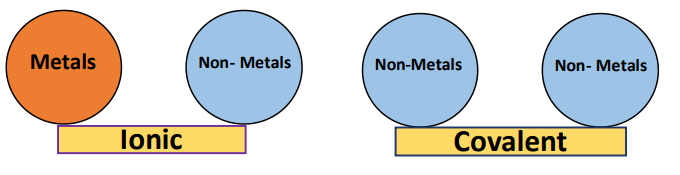
If a compound is formed by the combination of metal atoms with non-metal atoms, then it is an ionic compound. If a compound is formed by the combination of two or more non-metal atoms, then it is a covalent compound.
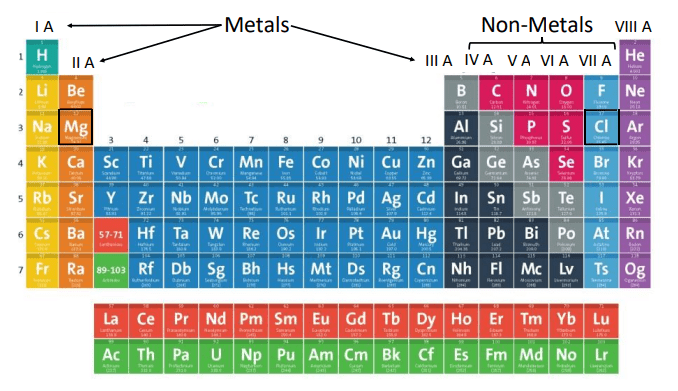
Commonly known metal atoms are situated in groups IA, IIA (s-block), and IIIA (p-block) of the Periodic Table. The metals present in Group IA are called alkali metals while the metals present in Group II A are alkaline earth metals.
On the other hand, non-metals can be located in groups IVA-VIIA (p-block) of the Periodic Table. Therefore, according to the definition given above,
If a compound is formed between a group IA element, for example, sodium (Na), and an element from group VIIA, for example, chlorine (Cl) then it is an ionic compound i.e., sodium chloride (NaCl), an ionic compound.
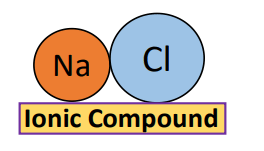
Similarly,
If a compound is formed between an element from group IVA (a non-metal group) for example a carbon (C) atom and atoms of an element from group VIA (another non-metal group) for example two atoms of oxygen (O) then it is a covalent compound i.e., carbon dioxide (CO2), is a covalent compound.
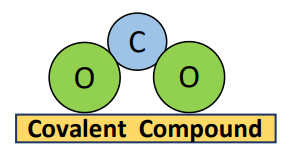
This concept applies to all other group combinations.
How to tell if a compound is ionic or covalent by its formula?
As we discussed above, the relevance of the group number of elements in determining whether something is ionic or covalent, additionally we can take some help from the formula of a chemical compound and use the Periodic Table to find its nature.
Let’s see how.
The formula of the chemical compound: MgCl2
- Step I: Identify the elements present in the compound. In this case, Mg and Cl.
- Step II: Locate Mg in the Periodic Table. It is in group IIA which is the metals group.
- Step III: Locate Cl in the Periodic Table. It is in group VIIA which is the non-metals group.
- Step IV: Identify the compound. It is formed by a metal and two non-metal atoms, so it is an ionic compound.
The formula of the chemical compound: SO2
- Step I: Identify the elements present in the compound. In this case, S and O.
- Step II: Locating from the Periodic Table, both S and O are in group VI A which is a nonmetals group so SO2 is a covalent compound.
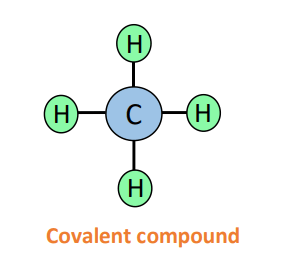
The formula of the chemical compound: CH4
In this case, the two elements involved are C and H. We are sure about carbon (C) that since it is in group IVA, so it is a non-metal. But for hydrogen (H) which is often placed above group IA of the Periodic Table, which is a metals group, we may be a little confused.
According to researchgate, “Hydrogen is most often classified as a nonmetal because it has many of the properties of nonmetals”.
Therefore, CH4 is also classified as a covalent compound.
Now that we know how to primarily identify a compound as either ionic or covalent, we are good to discuss what an ionic or a covalent bond is and how it is formed.
|
How to tell if a compound is ionic or covalent by its formula? – Key takeaways
|
Also, read in detail:
- Is NaCl ionic or covalent?
- Is NH3 ionic or covalent?
- Is H2S ionic or covalent?
- Is Na2O ionic or covalent?
- Is SO2 ionic or covalent?
- Is NO2 ionic or covalent?
- Is H2O ionic or covalent?
- Is MgO ionic or covalent?
- Is MgCl2 ionic or covalent?
How to determine if a bond is ionic or covalent?
Atoms use their valence shell electrons to form chemical bonds in order to gain a stable electronic configuration.
The chemical bonds are mainly classified as ionic or covalent depending upon how the atoms involved used their valence electrons.
What is an ionic bond?
An ionic bond is formed by the complete transference of valence shell electrons from one atom to the other.
An atom with an excess of electrons in its valence shell loses one or more of its electrons to form a positively charged ion called a cation.
Another atom with a deficiency of electrons in its valence shell gains the lost electrons to form a negatively charged ion called the anion.
Electrostatic forces of attraction develop between the oppositely charged ions called an ionic bond. An ionic bond is also known as an electrovalent bond.
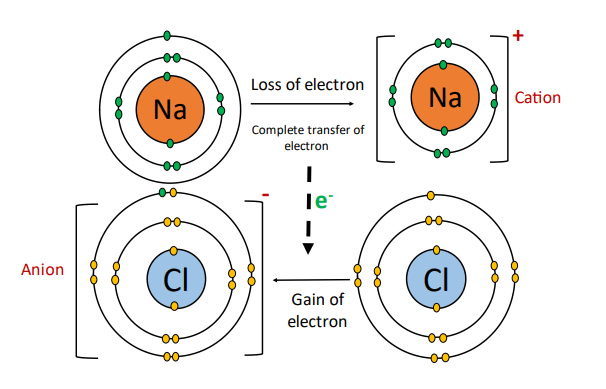
Metals have low ionization energy which is they have a tendency to lose electrons. While nonmetals have a high electron affinity/ electronegativity, so they tend to accept electrons readily.
In this way, metal and non-metal atoms form ionic bonds.
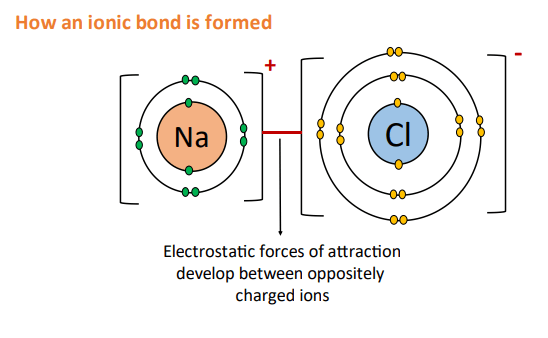
A large amount of energy is released when an ionic compound is formed known as the bond energy. Therefore, a large amount of energy needs to be supplied in order to break this bond.
Thus, an ionic bond is one of the strongest chemical bonds and ionic compounds are extremely stable compounds.
What is a covalent bond?
A covalent bond is formed by a mutual sharing of electrons between two or more non-metal atoms. In this way, all the atoms involved tend to obtain a stable noble gas electronic configuration.
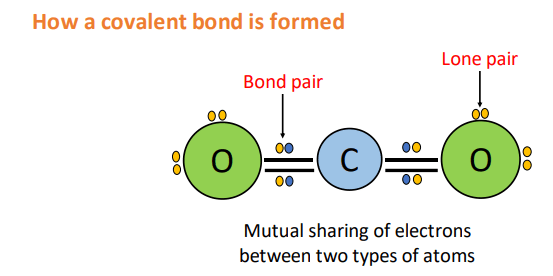
Each atom contributes one or more electrons to the shared electron cloud. In this way, the shared electron cloud is considered to belong equally to both atoms.
In the case of a covalent bond formed between atoms of two or more different elements, electronegativity counts as an important factor.
Electronegativity is the ability of an atom to attract a shared pair of electrons more strongly. Therefore, in a covalent bond, the more electronegative atom will more strongly attract the electron cloud and will gain a partial negative charge while the other atom will gain a partial positive charge.
How to know if a bond is ionic or covalent using electronegativity?
As we discussed already, an ionic bond is formed by a complete transference of electrons from one atom to another. Therefore, we know that there must be a large difference in electronegativity between the two atoms involved.
One atom should have a significantly high electron affinity than the others.

According to the Pauling scale of electronegativity-
- If the difference of electronegativity between the two atoms exists less than 1.7, then the bond formed between these atoms is covalent in nature.
- If the difference of electronegativity between the two atoms exists more than 1.7, then the bond formed between these atoms is ionic in nature.
Also, note that –
- A covalent bond, on the other hand, can be formed between identical or different atoms.
- Generally, if the electronegativity difference between atoms is less than 0.5 then it is non-polar covalent.
- If the electronegativity difference between atoms ranges from 0.5 to 1.7 then it is polar covalent.
|
How to know if a bond is ionic or covalent using electronegativity? |
||
| Electronegativity difference | Type of bond | Examples |
| 0.0 – 0.4 | Covalent (nonpolar) | Br2, H2 |
| 0.5 to 1.7 | Covalent (Polar) | NH3, H2O, etc. |
| >1.7 | Ionic | NaCl, MgO, etc. |
Some important concepts –
- Overall, a bond is covalent if atoms have an electronegativity difference of less than 1.7.
- Conversely, if the electronegativity difference lies between 1.7 to 2.0 and metal is involved then the bond is considered ionic. If only non-metals are involved then the bond is polar covalent.
- But still, the role of molecular geometry and the shape of a covalent molecule cannot be neglected in determining its polarity.
A polar covalent bond can also be recognized as an intermediate between a purely ionic bond and a purely covalent bond.
How to know if a covalent bond is polar or nonpolar?
As we already discussed –
- Generally, if the electronegativity difference between atoms is less than 0.5 then it is non-polar covalent.
- If the electronegativity difference between atoms ranges from 0.5 to 1.7 then it is polar covalent.
A chlorine molecule (Cl2) formed by the covalent bonding of two chlorine atoms is non-polar because the electronegativity difference between the two atoms is zero.
A sulfur dioxide (SO2) molecule formed by covalent bonding between sulfur and two oxygen atoms is polar because of the difference in electronegativity between the two different atoms involved.
⇒ The electronegativity value for sulfur atom = 2.58
⇒ The electronegativity value for oxygen atom = 3.4
∴ The difference in the electronegativity between sulfur and oxygen atoms = 0.82 [∴ polar covalent range = 0.5 to 1.7]
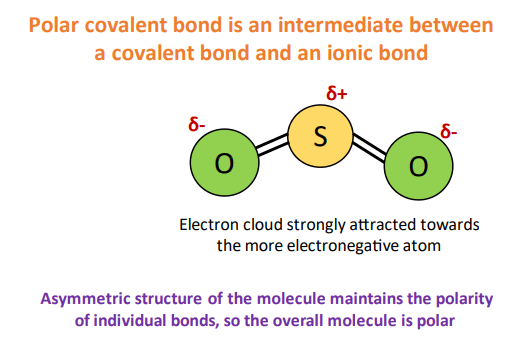
Same as, carbon dioxide (CO2) is formed by polar covalent bonds because of the difference in electronegativity between the two different atoms involved, however, it is an overall non-polar molecule.
This is because of the symmetric, geometrical structure of CO2
The bonds present in CO2 is polar covalent because of the electronegativity difference between carbon and oxygen.
⇒ The electronegativity value for carbon atom = 2.55
⇒ The electronegativity value for oxygen atom = 3.4
∴ The difference in the electronegativity between carbon and oxygen atoms = 0.85 [∴ polar covalent range = 0.5 to 1.7]
but whole molecule of CO2 is nonpolar in nature.

Check in details –
- A covalent bond formed by the mutual sharing of one electron pair i.e., a single electron contributed by each atom is called a single covalent bond.
- Contrarily, covalent bonds formed by the mutual sharing of two and three electron pairs are called double and triple covalent bonds respectively.
- Other than the shared pair of electrons, other electron pairs may also be present on the combined atoms. These are called nonbonding electrons or lone pair electrons.
A covalent bond is usually much weaker than an ionic bond. Covalent compounds thus have lower melting and boiling points than ionic compounds in their simple molecular forms.
However, a large number of covalently bonded molecules can combine together to form giant molecular lattices.
Diamond is a giant molecular lattice formed by covalently bonded carbon atoms.
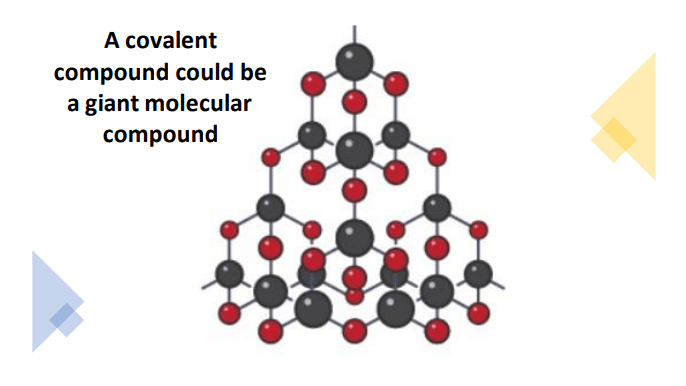
How to tell if the substance is ionic or covalent from its properties?
| Property | Ionic | Covalent |
| Stability | Ionic compounds are more stable than covalent compounds |
Covalent compounds are comparatively less stable than ionic compounds |
| Physical state | Solid at room temperature, for example – NaCl (s) |
Liquid or gas in the room temperature for example Br2 (l) or CO2 (g) |
| Melting and boiling points | High | Low |
| Solubility in water | High | Low |
| Electrical and thermal conductivity |
Poor conductor in solid-state. Conducts electricity in molten and aqueous states due to the presence of freely moving ions |
Does not conduct electricity or heat in any form. No freely moving ions or electrons present |
| Tensile strength | Hard and brittle | Usually soft and flexible |
| Chemical reactivity | Usually reacts faster | Slow to react |
| Examples | NaCl, MgO, MgCl2, etc. | NH3, SO2, NO2, etc. |
How to know if a compound is ionic or covalent according to Fajan’s rule?
Remember when we said at the beginning of this article that we cannot straightforwardly declare whether a compound is ionic or covalent?
Fajan’s rule says that the nature of specific chemical bonds can change from ionic to covalent even after the bond is formed.
Fajan rule’s are used to predict whether a chemical bond will be covalent or ionic, and depend on the charge on the cation and the relative sizes of the cation and anion.
Based on the Fajan rule, the ionic or covalent compounds can be distinguished by the following characteristics.
| Ionic characteristics | Covalent characteristics |
| Small anion | Large anion |
| Low charge | High charge |
| Large cation | Small cation |
Point to remember while using the Fajans‘ rule to predict the covalent or ionic character:
- The Fajans’ rule states that a compound with a large anion, large charge, and small cation will adopt the covalent character whereas a compound with a small anion, small charge, and large cation will adopt an ionic character.
- Fajans’ rule depends on the size of the relative size of cation and anion for predicting the ionic or covalent character in the molecule.
- The smaller the size of the cation and the larger the size of the anion, the greater the covalent character of the molecule.
Most important: The cation with a high positive charge will attract the electron in the outermost shell of the anion towards itself such that the electron cloud of the anion is distorted, hence, the electron is drawn into the space between positive cation and negative anion, and these electrons get shared between cation and anion. Therefore, the compound exhibit a covalent character.

Earlier we discussed cations and anions with reference to an ionic bond formation. For example, aluminum chloride (AlCl3) is formed by the combination of a metal (Al) from group IIIA and a non-metal (Cl) from group VIIA, the halogens group.
Al loses three electrons to form an Al3+ cation while three Cl atoms, each gain 1 electron to form a Cl– anion. So ideally, electrostatic forces of attraction develop between the oppositely charged ions, and an ionic bond is formed.
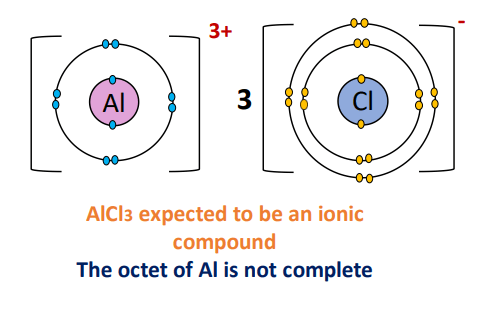
But Fajan’s rule says that AlCl3 is actually not an ionic but a covalent compound. Isn’t that contradictory? Let’s find out why.
Now, we will try to know Why AlCl3 has a covalent character according to the Fajans’ rule.
- AlCl3 has two ions, Al3+ as the cation and Cl– as the anion. The Al3+ is very small and highly charged, therefore, has a high charge density. The Cl– is a very large anion compared to Al3+ and has a very low charge density.
- Al3+ has a very high polarizing power since it is highly charged, so, when it comes close to the Cl–, then it polarizes the electron cloud of Cl– to the large extent.
- After polarizing the electron cloud of Cl– by the Al3+, the symmetrical shape of the Cl– anion gets distorted and electrons are drawn into the space between Al3+ and Cl–.
- The electrons that are drawn into the space between cation(Al3+) and anion(Cl–) are gets shared to complete their octet, hence, the bond formed between the ions of AlCl3 is exhibited great covalent character.
In short, “Al3+ is highly charged in nature and it can polarise the electron clouds of Cl– to a large extent. So, electrons get shared between the two ions. Hence the AlCl3 compound is a covalent one”

Although, AlCl3 is formed from metal and nonmetal and many of you assumed that it is an ionic compound but the bond formation in these types of compounds such as AlCl3, AlF3, AlBr, etc are best explained by the Fajans’ rule.
- Some compounds are neither purely ionic nor covalent in nature. Ionic bonds can exhibit covalent characteristics according to Fajan’s rule.
- Small-sized cations with high charge density can easily polarize large anions and change a bond’s nature from ionic to covalent such as in AlCl3.
In short, we can’t predict for some compound whether it is really ionic or covalent by only looking at its chemical formula or electronegativity difference, sometime, we have to taken the consideration of Fajan’s rule concept.
Please watch this video to understand this.
FAQ
Why is AlCl3 covalent while AlF3 is ionic? |
|
As Fajans‘ rule said, the covalent character increase when the size of the cation is small and the size of the anion is large, so that, the anion easily gets polarized by the cation, and electrons get shared between them. So, in the case of AlCl3 and AlF3, the cation(Al3+) is the same for both compounds, hence, just ignore it. Now see the size of the anion of both compounds. Clearly, the Size of Cl– > size of F– Hence, chlorine gets easily polarised by the Al3+ but on the other hand, the fluorine size is so small that Al3+ is not able to polarize it to such a great extent. Therefore, we can say, due to the size difference of anions, AlCl3 is covalent while AlF3 is ionic. |
Arrange the following according to the increasing order of their covalent character?
|
|
According to Fajan’s rule – the smaller cation and the largest anion should technically be the most covalent. So, in the above compounds, the cation is the same for all, therefore, check the size of the anion, larger the size of the anion higher the covalent character. Size of anion = I– > Br– > Cl– > F– Therefore, the highest covalent character order of these given compounds are – NaF < NaCl < NaBr < NaI. |
Identify Na2SO4 as either ionic or covalent? |
|
Na2SO4 is an ionic compound. It is formed by the combination of a metal (Na) with non-metal (S and O) atoms. It is formed by the complete transference of electrons from two Na+ ions to a sulfate (SO42-) ion. |
Identify NH4Cl as either ionic or covalent? |
|
NH4Cl is an ionic compound. It is a special case. Although it is composed of all non-metal atoms. But it is formed by a complete transference of electrons from an ammonium (NH4+) ion to chloride (Cl–) ion, so it is ionic. |
Using electronegativity, predict what type of bond is formed between C and an O atom. |
|
⇒ Electronegativity of carbon = 2.55 ⇒ Electronegativity of oxygen= 3.44 ∴ Electronegativity difference = 3.44 -2.55 = 0.89. It lies between 0.5 and 1.7 so a bond between a C and an O atom is polar covalent. |
Why is CO2 a non-polar covalent compound but SO2 is polar covalent? |
|
Both CO2 and SO2 are formed by the mutual sharing of electrons involving non-metal atoms so they are covalent molecules. CO2 has a linear, symmetrical geometry so the polarities of both C=O bonds get canceled in opposite directions and it is non-polar. SO2 is an asymmetric molecule so it is polar. |
Summary
- Ionic compounds are usually formed between a metal and non-metal atoms. And covalent compounds are usually formed between non-metal atoms.
- The most valid concept is that compounds formed by the complete transference of electrons are ionic while covalent compounds are formed by a mutual sharing of electrons.
- Covalent molecules can be purely covalent, non-polar in nature, or polar depending upon the difference in electronegativity and the molecular geometry of the molecules.
- If the difference of electronegativity between the two atoms exists less than 1.7, then the bond formed between these atoms is covalent in nature.
- If the difference of electronegativity between the two atoms exists more than 1.7, then the bond formed between these atoms is ionic in nature.
- Fajan rules are used to predict whether a chemical bond will be covalent or ionic, and depend on the charge of the cation and the relative sizes of the cation and anion.
About the author
Ammara Waheed is a highly qualified and experienced chemist, whose passion for Chemistry is evident in her writing. With a Bachelor of Science (Hons.) and Master of Philosophy (M. Phil) in Physical and Analytical Chemistry from Government College University (GCU) Lahore, Pakistan, with a hands-on laboratory experience in the Pakistan Council of Scientific and Industrial Research (PCSIR), Ammara has a solid educational foundation in her field. She comes from a distinguished research background and she documents her research endeavors for reputable journals such as Wiley and Elsevier. Her deep knowledge and expertise in the field of Chemistry make her a trusted and reliable authority in her profession. Let's connect - https://www.researchgate.net/profile/Ammara-Waheed