Propane (C3H8) + Oxygen (O2) = CO2 + H2O - How to balance?
To balance a chemical equation, the number of atoms of each element must be equal on both sides(reactant and product sides) of the equation.
Here is a step-by-step process to balance the equation “C3H8 + O2 → CO2 + H2O”:
Steps to balance the equation “C3H8 + O2 → CO2 + H2O”
Step 1: Write the unbalanced equation: ⇒ C3H8 + O2 → CO2 + H2O |
Step 2: Count the number of atoms of each element on both sides of the equation. Look at the number of atoms of each element on the reactant side (left side) and compare it to the number of atoms of that element on the product side (right side).
|
||||||||||
Step 3: Balance the Carbon atoms In the above equation, we can see that we have 3 Carbon atoms on the reactant side and 1 Carbon atom on the product side. So, to balance the number of Carbon atoms, we need to multiply the number of CO2 on the product side by 3. The new equation will be: ⇒ C3H8 + O2 → 3CO2 + H2O Again compare the number of atoms on the reactant side to the product side.
[∴ Carbon atoms balanced in the above equation] |
||||||||||
Step 4: Balance the Hydrogen atoms Similarly, according to the above equation(step 3), we have 8 Hydrogen atoms on the reactant side and 2 Hydrogen atoms on the product side. So, to balance the number of Hydrogen atoms, we need to multiply the number of H2O on the product side by 4. Now the equation looks like this: ⇒ C3H8 + O2 → 3CO2 + 4H2O Again compare the number of atoms on the reactant side to the product side.
[∴ Hydrogen atom balanced in this equation] |
||||||||||
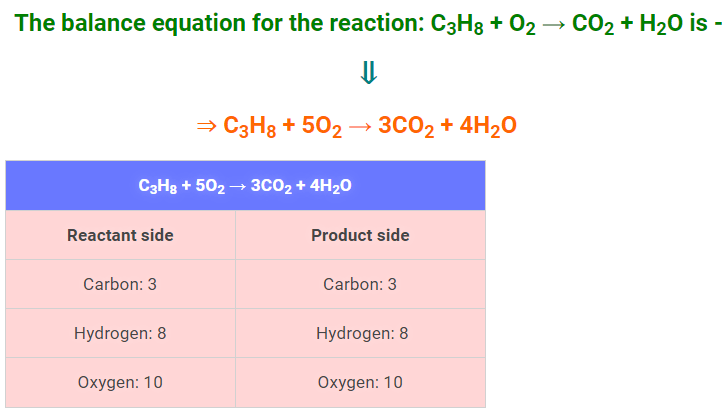
Step 5: Balance the Oxygen atoms Now we have to balance the Oxygen atoms, according to the Step 4 equation, we have 2 Oxygen atoms on the reactant side and (6 + 4 = 10) Oxygen atoms on the product side. So, we need to multiply the oxygen atoms on the reactant side by 5 to balance the number of Oxygen atoms. The final balanced equation will be: ∴ C3H8 + 5O2 → 3CO2 + 4H2O Again compare the number of atoms on the reactant side to the product side.
[∴ All the atoms balanced in this equation] |
||||||||||
So, the balance equation of the reaction: C3H8 + O2 → CO2 + H2O is – ⇒ C3H8 + 5O2 → 3CO2 + 4H2O [Answer]
|
||||||||||
In this step-by-step solution, we have balanced the equation: (C3H8 + O2 → CO2 + H2O) by adjusting the coefficient of each element, ensuring that the number of atoms of each element is the same on both sides of the equation.
FAQ
What type of reaction is C3H8 + 5O2 → 3CO2 + 4H2O? |
The reaction of C3H8 (propane) and O2 (oxygen) to form CO2 (carbon dioxide) and H2O (water) is a combustion reaction. A combustion reaction is a type of chemical reaction in which a fuel (in this case, C3H8 or propane) reacts with an oxidizer (O2 or oxygen) to produce heat and light (in the form of a flame) as well as new products (CO2 and H2O). The balanced chemical equation for this reaction (C3H8 + O2) is C3H8 + 5O2 → 3CO2 + 4H2O. The overall equation represents the complete chemical change of the reactants into the products. In this reaction, C3H8 is the fuel, and O2 is the oxidizer. |
How many grams of O2 are needed to completely burn 33 g of C3H8? |
The balanced chemical equation for this reaction (C3H8 + O2) is – ⇒ C3H8 + 5O2 → 3CO2 + 4H2O. This equation tells us that for every 1 mole of C3H8 that is burned, 5 moles of O2 are consumed. To determine the number of grams of O2 needed to completely burn 33 grams of C3H8, we need to convert the mass of C3H8 to moles and then multiply by the stoichiometric coefficient of O2. First, we can convert the mass of C3H8 to moles using its molar mass, which is 44.1 g/mol. ⇒ Number of moles of C3H8 = Mass/molar mass = 33 g/44.1 g/mol = 0.747 mol C3H8 Then, we can multiply this value by the stoichiometric coefficient of O2 from the balanced equation to find the number of moles of O2 required. ⇒ 0.747 * 5 = 3.735 mol O2 Now, we can convert the number of moles of O2 back to grams using its molar mass, which is 32.0 g/mol. ⇒ 3.735 * 32.0 g/mol = 119.52 g O2 So, approximately 119.52 g of O2 is needed to completely burn 33 g of C3H8. |
What coefficient would the O2 have after balancing C3H8 + O2 → CO2 + H2O? |
As we already know, the balancing equation for the reaction (C3H8 + O2 → CO2 + H2O) is – ⇒ C3H8 + 5O2 → 3CO2 + 4H2O. According to the above equation, the coefficient of O2 would be 5. This means that for every 1 mole of C3H8 that is burned, 5 moles of O2 are consumed. |
How many moles of oxygen (O2) is necessary to react with 4 moles of propane (C3H8)? |
First, let’s start with the balanced chemical equation for the combustion of propane: ⇒ C3H8 + 5O2 → 3CO2 + 4H2O. This equation tells us that for every 1 mole of propane (C3H8) that is burned, 5 moles of O2 are consumed. We are given the 4 moles of Propane (C3H8) and we know that for every 1 mole of propane that is burned, 5 moles of O2 are consumed. So, to react with 4 moles of propane (C3H8), we would need – ∴ 4 moles of propane * 5 moles of O2/1 mole of propane = 20 moles of O2. So, the 20 moles of oxygen (O2) necessary to react with 4 moles of propane (C3H8). |
Here check the balancing equation (C3H8 + O2 → CO2 + H2O) video to clear your doubt –
References
- ChemicalAid. “Equation Balancer.” [Online]. Available: https://www.chemicalaid.com/tools/equationbalancer.php?equation=C3H8+%2B+O2+%3D+CO2+%2B+H2O&hl=en. [Accessed: 25-Jan-2023].
- Socratic. “Balance the equation: C3H8 + O2 = CO2 + H2O.” [Online]. Available: https://socratic.org/questions/balance-the-equation-c3h8-o2-co2-h2o. [Accessed: 25-Jan-2023].
About the author
Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. The team at Topblogtenz includes experts like experienced researchers, professors, and educators, with the goal of making complex subjects like chemistry accessible and understandable for all. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/
