CH3CH2CH2CH3 Lewis structure, Polar or nonpolar, Isomers, Acid or base, Intermolecular forces
What is butane (CH3CH2CH2CH3)?
Butane is a highly flammable, easily liquefiable, colorless gas at room temperature and pressure. It is represented by the chemical formula C4H10. However, the condensed structural formula for C4H10 is CH3CH2CH2CH3.
Thus, CH3CH2CH2CH3 represents the straight-chain structure of butane, also known as n-butane.
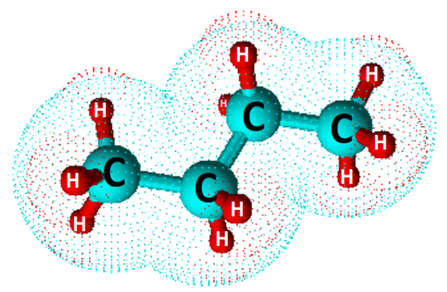
This condensed structural formula shows that butane is a saturated hydrocarbon made up of carbon (C) and hydrogen (H) atoms only. The carbon and hydrogen atoms are bonded to one another via single covalent bonds.
As per organic chemistry principles, n-butane (CH3CH2CH2CH3) is an aliphatic alkane where an alkane is defined as an acyclic hydrocarbon made up of C-C single bonds. Alkanes are represented by the general formula CnH2n+2 where n is the number of carbon atoms present in the straight-chain structure. As 4 carbon atoms are present in CH3CH2CH2CH3 so its molecular formula is C4H2(4) +2 = C4H10.
Other than its straight chain structure, there are other related structures of butane represented by the same molecular formula (C4H10) but a different structural formula. These structures are known as the isomers of butane.
You will find out about butane isomers, the Lewis structure of butane, its physicochemical properties, etc., all through this article. So continue reading!
| IUPAC name of the chemical compound | Butane |
| Common name | n-butane |
| Molecular formula | C4H10 |
| Condensed structural formula | CH3CH2CH2CH3 |
| Molecular geometry or shape | Tetrahedral |
| Hybridization | Sp3 |
| Molar mass | 58.122 g/mol |
| Boiling point | -0.5 ° C |
| Water solubility | 61 mg/L (at 20°C) |
| Chemical bonding | C-C and C-H covalent chemical bonds |
| Intermolecular forces of attraction | Weak intermolecular forces (London-dispersion forces) |
| Polar or non-polar | Non-polar molecule |
| Acid or base | Neither acidic nor basic |
CH3CH2CH2CH3 IUPAC name
The IUPAC name for the structure represented by CH3CH2CH2CH3 is butane.
The word butane is a combination of two words i.e., but and ane. ‘But’ means four. As there are 4 carbon atoms in CH3CH2CH2CH3 so ‘alk’ in alkane is replaced with the prefix ‘but’ and it gives us the IUPAC name ‘but-ane’ or butane.
The chemical compound is usually represented by n-butane where n signifies that it is a straight-chain alkane. The alphabet n is also sometimes used to represent normal butane because CH3CH2CH2CH3 is the most dominant isomer of butane. It is 99% present in a mixture of butane isomers.
Other names for butane that are rarely used are Diethyl Methylethylmethane and tetracarbane.
CH3CH2CH2CH3 structure
The condensed structural formula CH3CH2CH2CH3 can be expanded to obtain the structure of n-butane, as shown below.
The terminal carbon atoms are bonded to 1 C-atom and 3 H-atoms via single covalent bonds. The middle carbon atoms are bonded to 2 C-atoms and 2 H-atoms, each.
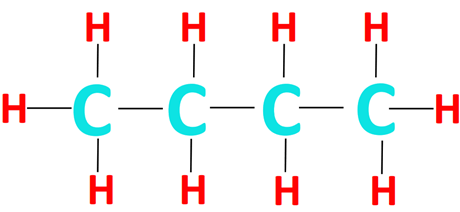
In this way, each C-atom forms a total of 4 covalent bonds which represents a complete octet electronic configuration. Conversely, each H-atom in the CH3CH2CH2CH3 structure forms only 1 covalent bond and achieves a complete duplet configuration.
There are a total of 13 single covalent bonds (sigma bonds) in the above structure.
CH3CH2CH2CH3 Lewis structure
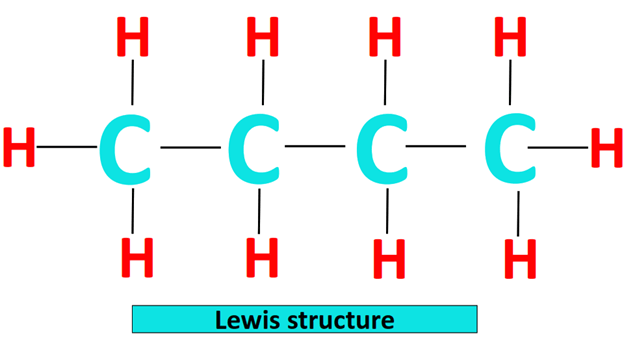
The Lewis structure of a molecule is a simplified representation of all the valence electrons present in it.
The Lewis structure of n-butane is shown below. Each straight line represents a single covalent bond containing 2 electrons. Thus, there are a total of 13(2) = 26 valence electrons in the Lewis structure of CH3CH2CH2CH3.
CH3CH2CH2CH3 intermolecular forces and physicochemical properties
There is a strong intramolecular covalent bonding between C-C and C-H atoms in the butane molecule. However, the intermolecular forces of attraction between individual CH3CH2CH2CH3 molecules are weak.
There is a negligible electronegativity difference between a C-atom and an H-atom while no electronegativity difference exists at all between two identical C atoms. Thus both C-C and C-H bonds are non-polar in the CH3CH2CH2CH3 molecules. Consequently, no permanent dipoles develop in the molecule neither a strong force of attraction exists between the molecules.
Temporary (instantaneous) dipoles develop due to the continuous motion of electrons around molecular nuclei. This results in London dispersion forces, the weakest intermolecular force of attraction between CH3CH2CH2CH3 molecules.
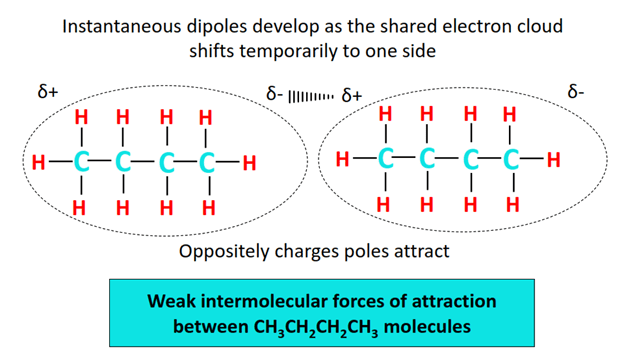
The boiling point of CH3CH2CH2CH3
It is due to the weak intermolecular forces of attraction that n-butane has an extremely low boiling point (-0.5°C) and a high vapor pressure (170 kPa) at room temperature.
CH3CH2CH2CH3 attractive forces can be easily overcome. Therefore, n-butane exists as a gas that quickly vaporizes and is highly liquefiable at the same time.
Solubility of CH3CH2CH2CH3
Water (H2O) molecules can break the temporary attractive forces between CH3CH2CH2CH3 molecules to a small extent therefore n-butane is slightly water-soluble. However only 61 mg n-butane dissolves per liter of water, this is because like dissolves like.
Water (H2O) is a polar molecule while n-butane is non-polar so water cannot completely dissolve CH3CH2CH2CH3 molecules. Rather, n-butane is highly soluble in organic solvents of like polarity such as n-hexane, ethanol, etc.
Similarly, n-butane can dissolve non-polar compounds in itself such as lipids, oils, and waxes.
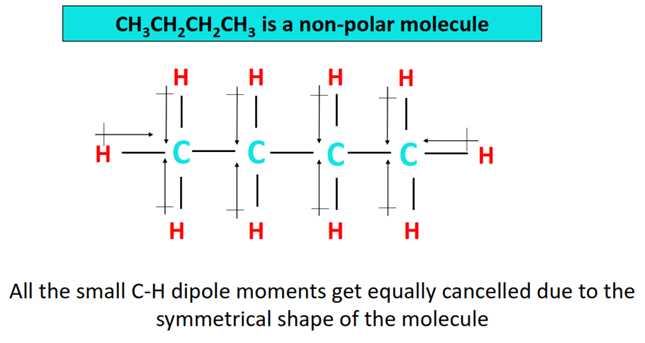
Is CH3CH2CH2CH3 polar or non-polar?
As already discussed, n-butane is a non-polar molecule. According to Pauling’s electronegativity scale, a chemical covalent bond is considered polar if the bonded atoms have an electronegativity difference between 0.5 to 1.6 units.
However, in CH3CH2CH2CH3 no electronegativity difference exists between the bonded C atoms in a C-C bond.
A small electronegativity difference of 0.35 units exists between a carbon (E.N = 2.55) and a hydrogen (E.N = 2.20) atom in a C-H bond in CH3CH2CH2CH3. 0.35 < 0.5 so the C-H bond is also only weakly polar.
But the small dipole moments (symbol µ) of C-H bonds eventually get canceled in the symmetrical structure of CH3CH2CH2CH3. The electron cloud stays uniformly distributed in the molecule overall thus n-butane is non-polar (net µ = 0).
Is CH3CH2CH2CH3 acid or base?
n-butane (CH3CH2CH2CH3) is a saturated hydrocarbon which means there are strong sigma bonds between the bonded C-C and C-H atoms in the continuous straight chain structure.
Consequently, no covalent bond can readily break nor can a new bond be formed easily. As the C-H bond cannot break easily so n-butane is clearly not a proton donor as expected of a Bronsted-Lowry acid.
Also, there are no delocalized electrons present on any atom in the CH3CH2CH2CH3 molecule. As per Lewis acid-base concept, acids are electron pair acceptors while bases are electron pair donors. This means the n-butane molecule is neither acidic nor basic.
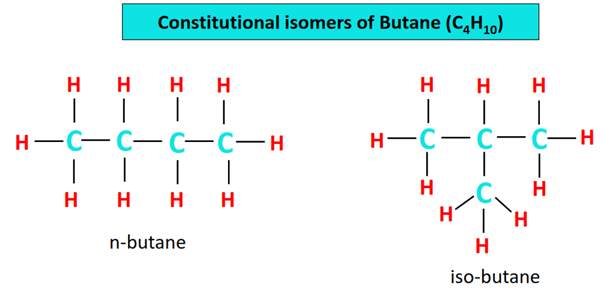
Butane (CH3CH2CH2CH3) isomers
Isomers are chemical compounds that have the same molecular formula but a different structural formula.
Other than the straight chain structure of n-butane, C4H10 can also be represented by a branched structure, as shown below. This is called iso-butane as it is an isomer of the normal butane. Its IUPAC name is 2-methyl propane.
n-butane and iso-butane are constitutional isomers.
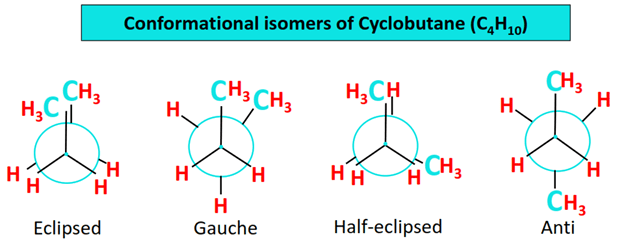
Butane also exhibits another type of isomerism known as conformational isomerism.
Conformational isomers also called conformers are stereoisomers i.e., they have the same molecular formula, and the same structural formula in 2 dimensions but their 3-dimensional arrangement in space differs.
Conformers can be interconverted just by the rotation of a single bond, without breaking and/or making a new bond.
There are 4 conformational isomers of butane, as shown below. You may notice that these conformers are cyclic structures unlike aliphatic isomers of butane. Therefore, these are called cyclobutanes.
FAQ
What is the name of the compound CH3CH2CH2CH3? |
CH3CH2CH2CH3 is the condensed structural formula for a chemical compound called n-butane. n-butane is a saturated hydrocarbon. It belongs to the alkane family of organic compounds. |
What is the molar mass of CH3CH2CH2CH3? |
The experimentally calculated molar mass of CH3CH2CH2CH3 or C4H10 is 58. 122 g/mol. However, its approximate molar mass can be calculated using the formula given below. Molar mass of C4H10 = 4 (Mass number of C) + 10 ( Mass number of H) = 4(12) + 1(10) = 58 g/mol. |
What is the IUPAC name for n-butane? |
The IUPAC name for n-butane is simply butane. |
What type of intermolecular forces of attraction exists between CH3CH2CH2CH3 molecules? |
| The weakest intermolecular forces of attraction are present between CH3CH2CH2CH3 molecules due to no permanent dipoles existing in the molecules. These are known as London dispersion forces. |
Is CH3CH2CH2CH3 polar or non-polar? |
CH3CH2CH2CH3 is a non-polar molecule overall due to a negligible to very small electronegativity difference between the bonded C-C and C-H atoms respectively. Furthermore, the dipole moments of weakly polar C-H bonds get canceled completely in the symmetrical molecular arrangement of CH3CH2CH2CH3. |
How many isomers are there of CH3CH2CH2CH3? |
| There are 2 isomers i.e., the straight chain structure n-butane and the branched structure iso-butane. So CH3CH2CH2CH3 has one isomer (other than itself), represented by (CH3)2CHCH3. |
How many total isomers are there of butane? |
| The butane molecule represented by the molecular formula C4H10 possesses 2 constitutional isomers and 4 conformational isomers which makes a total of 6 isomers. |
Is CH3CH2CH2CH3 soluble in water? |
| CH3CH2CH2CH3 is only slightly water-soluble due to its non-polar nature. Like dissolves like so polar water molecules cannot very easily dissolute non-polar CH3CH2CH2CH3 molecules. |
Is CH3CH2CH2CH3 acid or base? |
CH3CH2CH2CH3 is a neutral molecule. It is neither an acid nor a base. Strong covalent bonding and absence of non-bonded or pi-bonded delocalized electrons in CH3CH2CH2CH3 molecules denotes it is neither a proton donor or acceptor nor an electron pair donor or acceptor. |
Is CH3CH2CH2CH3 optically active? |
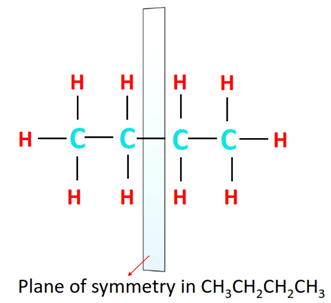
Optically active compounds are asymmetric molecules that rotate the plane of vibration of plane-polarized light in a specific direction. A plane of symmetry is present in n-butane and there is no chiral carbon (carbon bonded to 4 different groups) in it. Thus, CH3CH2CH2CH3 is optically inactive.
|
Summary
- CH3CH2CH2CH3 is the condensed structural formula for n-butane.
- n-butane is the naturally abundant, straight chain isomer of butane (molecular formula = C4H10, molar mass = 58.122 g/mol).
- CH3CH2CH2CH3 exists as a colorless gas with a gasoline-like odor at r.t.p.
- Strong single covalent bonds exist between C-C and C-H bonded atoms in CH3CH2CH2CH3.
- Weak intermolecular forces of attraction are present between CH3CH2CH2CH3 molecules therefore it has a low boiling point (-0.5°C).
- CH3CH2CH2CH3 is a non-polar molecule.
- It is only slightly water-soluble but highly miscible with other non-polar organic compounds.
- Iso-butane is the branched isomer of butane.
About the author
Ammara Waheed is a highly qualified and experienced chemist, whose passion for Chemistry is evident in her writing. With a Bachelor of Science (Hons.) and Master of Philosophy (M. Phil) in Physical and Analytical Chemistry from Government College University (GCU) Lahore, Pakistan, with a hands-on laboratory experience in the Pakistan Council of Scientific and Industrial Research (PCSIR), Ammara has a solid educational foundation in her field. She comes from a distinguished research background and she documents her research endeavors for reputable journals such as Wiley and Elsevier. Her deep knowledge and expertise in the field of Chemistry make her a trusted and reliable authority in her profession. Let's connect - https://www.researchgate.net/profile/Ammara-Waheed