Is Methanol (CH3OH) an acid or base?

Methanol is also known as methyl alcohol or carbinol has a chemical formula CH3OH or CH4O. It appears as a colorless liquid and has a sweet + pungent odor. It is completely miscible in water. It is used as a solvent for paints and plastics.
In this article, we will discuss Is Methanol (CH3OH) an acid or base?
So, Is CH3OH an acid or base? Methanol (CH3OH) is considered acidic as well as basic, depending on what it is reacting with. When it acts as an acid, it makes the conjugate base (CH3O–) and when it acts as a base, it makes conjugate acid (CH3OH2+)
| Name of Molecule | Methanol |
| Chemical formula | CH3OH |
| Molar mass | 32.04 g mol−1 |
| Nature | Both acidic and basic |
| The conjugate acid of CH3OH | CH3OH2+ |
| The conjugate base of CH3OH | CH3O– |
Why CH3OH act as acid?
An acid is a substance that releases an H+ ion in water or “donates a proton” and having a pH value from 1 to 6.
To understand whether CH3OH is an acid or base, look out for the two important theories of the acid-base concept (a). Arrhenius theory (b). Bronsted-Lowry theory
1. Arrhenius’s theory for acid:
According to Arrhenius’s theory for acid, the substance which produces an H+ ion on dissolving in an aqueous solution is categorized as an acid.
So, “the CH3OH compound has one hydrogen atom attached to the oxygen atom which can be dissociated as H+ ions in the aqueous solution”.
⇒ CH3OH(aq) ⇌ H+ + CH3O−
Therefore, we can say CH3OH is acid as per Arrhenius’s theory of acid-base pair.
2. Bronsted-Lowry theory for acid:
According to Bronsted-Lowry’s theory for acid, the substance which donates the proton to other compounds and itself makes a conjugate base categorized as an acid. So, when CH3OH reacted with NH3, it donates the proton to NH3 and itself makes a conjugate base(CH3O–).
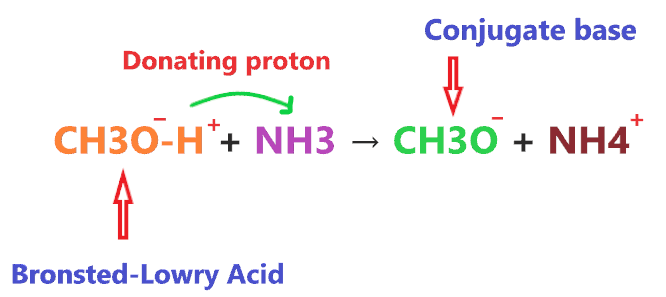
As you see in the above reaction, CH3OH donates the one H+ ion to the NH3 molecule and forms a conjugate base (CH3O–).
Note: NH3 is a weak base in nature but it is more basic than CH3OH, hence, it will accept the proton from CH3OH.
Hence, in the above reaction, CH3OH will act as a Bronsted-Lowry acid as it is “donating the proton” and NH3 will act as a Bronsted-Lowry base as it “accepts the proton”.
Also check:-
Why CH3OH act as base?
A base is a compound that has a pH value of more than 7 and can release hydroxide ions (OH–) in an aqueous solution or Base is defined as a “Proton acceptor”.
Now, many of you assume that CH3OH contains an OH group and it must be basic, well, it is not, “A base is a compound that dissociates into some hydroxide ions(OH–)”, hence, it is not necessary is any compound contains OH group will be considered as a base.
The compound should furnish some OH– ions in order to consider as basic or it should accept the proton from another compound as per the definition of Bronsted-Lowry base.
“It is not the OH hydroxy group that is basic but the OH– hydroxide ion. Only those compounds that dissociate into some cation and a hydroxide ion can be considered basic”.
So, Why does CH3OH act as a base? CH3OH acts as a base when reacting with the compound that is more acidic than it, i.e. HCl, HNO3, H2SO4, etc. This is because a stronger acid has more ability to “donate the proton”.
Like when CH3OH reacts with HCl, it acts as a base, this is because HCl is stronger acid and has the ability to donate a proton very easily.
Therefore, when CH3OH reacts with stronger acid like HCl, it is left with no choice and has to accept the proton from HCl. And we know anything that accepts the proton act as a base.
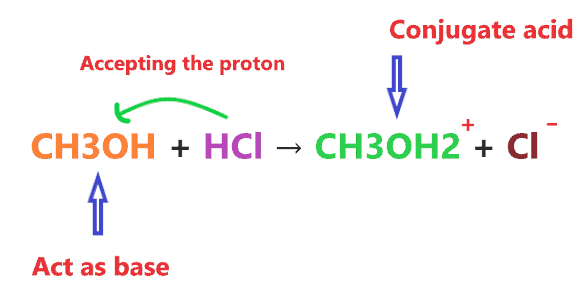
As we see in the above reaction, CH3OH reacts with a stronger acid(HCl) than it, therefore, accepting one proton from HCl and making a conjugate acid(CH3OH2+).
As per Bronsted-Lowry base theory, a compound is said to base when it accepts the proton from reacting species and forms a conjugate acid by adding one proton to itself.
Therefore, CH3OH can also act as a Bronsted-Lowry base when reacting with stronger acid than it such as HCl.
Is CH3OH Lewis acid or base?
Lewis’s theory is a very important acid-base theory to check whether a compound (CH3OH) acid or base?
According to the Lewis theory, a compound is said to be acid when it accepts the pair of electrons and a compound is said to be base when it donates the pair of electrons.
⇒ Lewis acid → lone pair acceptor
⇒ Lewis base → lone pair donator
Now, Is CH3OH Lewis acid or base? To know this first we should know what type of compound is Lewis acid and Lewis base.
Lewis acid: They are electron-loving compounds and are also known as electrophilic compounds that accept the electron pair from Lewis bases.
Some examples of Lewis acids that can accept the pair of electrons from another species.
- The molecules or ions with an incomplete octet of electrons. Example – BF3, BCl3, AlF3, etc.
- The molecule that central atom has an empty d-orbital. Example- SiCl4 (1s22s22p63s23p2 3d0).
- The compounds that the central atom formed multiple bonds with the adjacent atoms. Example – SiO2 (O = Si = O), CO2, SO2, etc.
- Simple cations like H+, Na+, Mg+2, Al+3, etc. are lewis acids since they are able to accept the electrons. ( Exception NH4+, PH4+ are not a lewis acid)
Lewis base: They are electron-rich species and also known as nucleophile compounds that donate the electron pairs to the Lewis acids.
Some examples of Lewis bases that can donate the pair of electrons to other species.
- The negative ion such as H–, OH–, F–, etc.
- Species that have lone pair of electrons. Example – NH3, H2O, CH3–, etc.
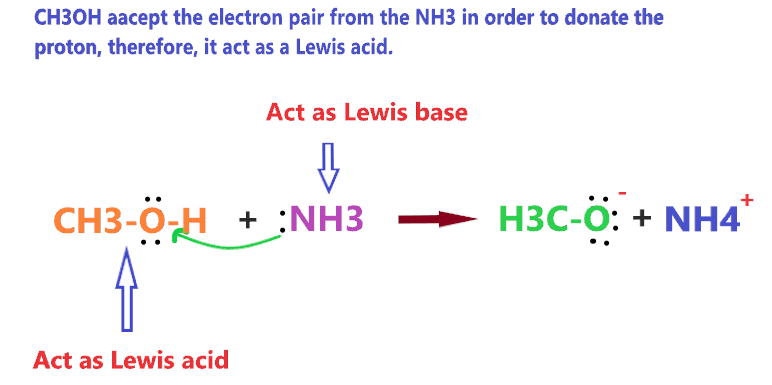
CH3OH can act as Lewis acid when it reacts with the Lewis base such as NH3 because CH3OH has to accept the electron pair from the NH3 in order to donate the proton. It’s a “trade”.
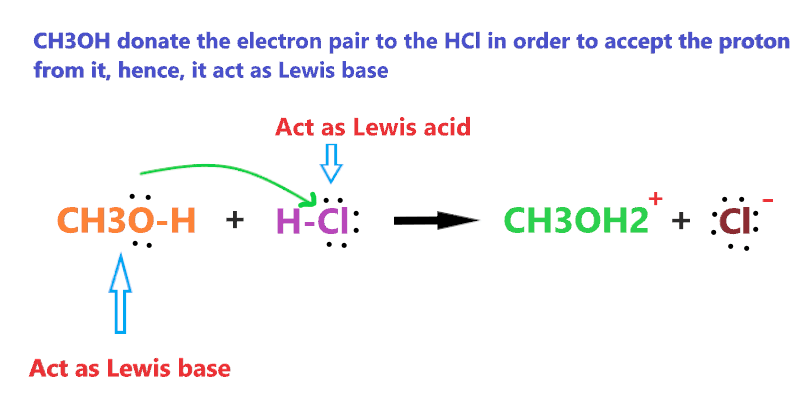
CH3OH can also act as a Lewis base when it reacts with the Lewis acid such as HCl because CH3OH has to donate the electron pair to the HCl in order to accept the proton from it.
Also read:-
Uses of Methanol
- It is used in the manufacture of formaldehyde and acetic acid.
- It is used as a solvent for paints and plastics.
- It is used in the manufacturing or synthesis of chemicals.
- It is used to remove water from aviation fuels.
- It is used in wastewater plants.
- It is used as a substitute for gasoline in automobile vehicles.
- It is used as a fuel for internal combustion engines.
Properties of Methanol
- It is the simplest alcohol that appears as a colorless fairly volatile liquid.
- It is a volatile and flammable liquid with an odor similar to ethanol or ethyl alcohol.
- It has a boiling point of 64.7 °C and a melting point of −97.6 °C.
- It is miscible in water and has a dipole moment of 1.69 D.
- It is extremely poisonous and flammable.
- “It is not a hydrocarbon since the hydroxyl group is chemically bonded to the carbon atom.”
- Methanol vapors are slightly heavier than air, can travel and ignite.
Summary
- The conjugate acid of CH3OH is Methyloxonium(CH3OH2+).
- The conjugate base of CH3OH is a Methanolate(CH3O–).
- Is Methanol (CH3OH) acid or base? CH3OH (Methanol) is both an acid and base. Since it can act as an acid in presence of a strong base than it such as NH3 and act as a base in presence of strong acid such as HCl.
- CH3OH can act as Lewis acid as well as Lewis base
- The acid dissociation constant value(Ka) for CH3OH is approx 3.2 × 10-16 which is way lower than 1, hence, CH3OH is a weak acid in nature.
About the author
Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. The team at Topblogtenz includes experts like experienced researchers, professors, and educators, with the goal of making complex subjects like chemistry accessible and understandable for all. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/

