Is Carbonic (H2CO3) an acid or base? Weak or Strong - Conjugate base

Carbonic acid is a hypothetical acid, composed of carbon dioxide and water having the chemical formula H2CO3. It is a dibasic acid because it contains two replaceable hydrogen atoms. It has a role as a mouse metabolite
In this article, we will discuss Is Carbonic (H2CO3) an acid or base? Is it strong or weak in nature? Its conjugate acid-base pairs, etc.
So, Is H2CO3 an acid or base? H2CO3 is considered an acid. Because on dissolving in an aqueous solution, it dissociates into two ions (H+ and HCO3–) and anything that furnishes proton ion in a solution is considered as the acid in nature.
| Name of Molecule | Carbonic acid |
| Chemical formula | H2CO3 |
| Molar mass | 62.02 g/mol |
| Conjugate base | HCO3– |
| Nature | Weak acid |
Why H2CO3 is acid?
An acid is a substance that liberates an H+ ion or proton when dissolved in water and having a pH value from 1 to 6.
So, H2CO3 is considered an acid because when it is dissolved in water or an aqueous solution then it dissociates into two parts (HCO3– and H+), the presence of the H+ ion in the aqueous solution of H2CO3 makes it acid in nature.
⇒ H2CO3 ⇌ H+ + HCO3−
To understand whether H2CO3 is an acid or base, look out the two important theories of the acid-base concept (a). Arrhenius theory (b). Bronsted-Lowry theory
1. Arrhenius’s theory for acid:
According to Arrhenius’s theory for acid, the substance which produces an H+ ion or proton on dissolving in an aqueous solution is categorized as an acid. So, when H2CO3 is dissolved in an aqueous solution then it produces some H+ ion or proton in the solution.
Therefore, we can say H2CO3 is acid as per Arrhenius’s theory of acid-base pair.
2. Bronsted-Lowry theory for acid:
According to Bronsted-Lowry’s theory for acid, the substance which donates the proton to other compounds and itself makes a conjugate base categorized as an acid. So, when H2CO3 is treated with water, it donates the proton or H+ ion and itself makes a conjugate base(HCO3–).
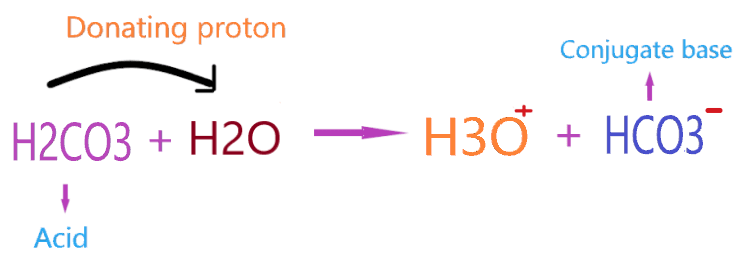
As you see in the above reaction, H2CO3 liberates or donates the one H+ ion to the water molecule and itself makes a conjugate base (HCO3–). The final aqueous solution of H2CO3 contains H3O+ ions and a conjugate base.
Therefore, we can say H2CO3 acts as Bronsted-Lowry acid because of proton donating ability when reacting with a compound such as H2O.
Note:- Bronsted-Lowry theory is not limited to an aqueous solution, this theory state that a substance is said to be Bronsted-Lowry acid when it donates the proton to other reacting species, the reacting species should be base or very less acidic in nature because according to this theory-
A substance can function as an Bronsted-Lowry acid only in the presence of a base. i.e. OH–, NaOH, NH3, H2O, etc.
But Arrhenius’s theory said a substance is an acid when it loses the proton on dissolving in an aqueous solution. The presence of water solution is a must for knowing the acidic nature of the substance according to Arrhenius’s theory.
Also Read: – Is HCO3– an acid or base?
Is H2CO3 strong acid or weak acid?
For knowing whether carbonic acid is strong or weak, look out the basic difference between a strong acid and weak acid-
A strong acid is a compound that is 100% ionized or completely dissociates in a solution and gives a high amount of hydrogen ions. It means all the moles of acid will dissociate to give H+ ions and no undissociated acid remains in the solution. They have a low pH value and good electrical conductivity properties.
Examples of strong acids: Hydrochloric acid(HCl), Sulfuric acid(H2SO4), Nitric acid(HNO3), HBr, etc.
Also Read:-
- Is H2SO4 a strong acid?
- Is HNO3 a strong acid?
- Is HCl a strong acid?
- Is HI a strong acid?
- Is HClO4 a strong acid?
- Is HBr a strong acid?
And weak acid is a compound that partially dissociates which means not all moles of weak acid dissociate in a solution to give H+ ion or they are not 100 % ionized in a solution and give only a low amount of hydrogen ions. They have a high pKa or pH value with moderate electrical conductivity property and weak electrolyte compared to strong acid.
Examples of a weak acids – Hydrogen cyanide(HCN), Ammonium ion(NH4+), Phosphoric acid(H3PO4), HF, etc.
Also Read:
- Is CH3COOH a weak acid?
- Is HF a weak acid?
- Is HCN a weak acid?
- Is HNO2 a weak acid?
- Is HCOOH a weak acid?
- Is H3PO4 a weak acid?
- Is NH4+ a weak acid?
| Strong acid | Weak acid |
| They ionize completely. | They do not completely ionize. |
| They have high conductivity. | They have less conductivity. |
| The value of pH lies between 0 to 3. | The value of pH lies between 3 to 7. |
| They dissociate completely to produce an H+ ion. | They dissociate partially to produce an H+ ion. |
| They are strong electrolytes. | They are weak electrolytes compared to strong acids. |
| Example – HCl, HNO3, HBr, etc. | Example – HNO2, HCOOH, CH3COOH, etc. |
Now, Is carbonic acid (H2CO3) strong or weak? H2CO3 is a weak acid. Because it partially dissociates in an aqueous solution or it does not ionize completely in solution to yield H+ ions. The strength of an acid depends on its ability to ionize completely or partially in a solution.
So, all molecules of carbonic acid not ionized completely cause fewer hydrogen ions in the aqueous solution and we know the more the hydrogen ion present in the solution, the higher will be the acidic strength of that solution.
The carbonic acid dissociates in two steps, first, it breaks into hydrogen carbonate ion(HCO3–) then again, it HCO3– ions dissociate into carbonate ion(CO32-).
⇒ H2CO3 + H2O ⇔ H3O+ + HCO3− [Partially dissociate]
⇒ HCO3− + H2O ⇔ H3O+ + CO32- [Partially dissociate]
∴ The double arrow in the reaction of H2CO3 and water shows that both forward and backward reactions will occur at equilibrium.
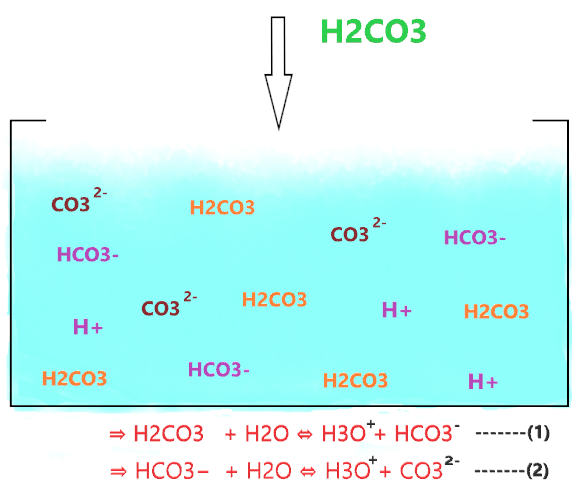
As shown in the figure, when H2CO3 is dissolved in water, it partially dissociates into ions (HCO3– and H+) but the ion(HCO3–) is not so stable, hence, it again reforms with H+ and forms H2CO3. Some of the HCO3– ions also release H+ ions due to their weakly acidic nature and form CO32- ions.
So, not all the H2CO3 molecules dissociate, most of them stay together and only a few of them dissociate and liberate H+ ions. And those H2CO3 molecules dissociate, their most of the split ions(H+ and HCO3–) again reform with each other and are converted into the original H2CO3 molecule.
This causes a lower amount of hydrogen ions in the aqueous solution of H2CO3 which ultimately makes it a weak acid in nature.
The acid dissociation constant value(Ka) for H2CO3 is 4.2 × 10-7 and anything less than 1 value of the acid dissociation constant for the molecule shows a weakly acidic nature.
⇒ If Ka >> 1, then the acid is strong in nature.
⇒ If Ka << 1, then the acid is weak in nature.
Note:- In weak acid, the backward is more dominating than forwarding reaction, as the splitting ions easily react with each other to reform the acid molecule.
Also Read:
Here’s the list of some common acids and bases with their strength.

Why H2CO3 is not a base?
A base is a substance that accepts the proton from other compounds or releases OH– ion in an aqueous solution. The strength of basicity in an aqueous solution depends on the number of OH– ions in the solution.
So, H2CO3 is not a base because when it is dissolved in water, it produces an H+ ion instead of an OH– ion. Also, H2CO3 is a proton donor which is also against the property of the base.
⇒ H2CO3 + (aq) → H+ (aq) + HCO3– (aq)
Here’s what Arrhenius and Bronsted-Lowry said for the base compound-
Arrhenius’s theory for base:
According to the Arrhenius theory for the base, the substance which produces an OH- ion on dissolving in an aqueous solution is categorized as a base. H2CO3 doesn’t liberate any OH– ion when dissolved in water, so it does not fall into the category of Arrhenius bases.
Bronsted-Lowry theory for base:
According to the Bronsted-Lowry theory for the base, the substance which accepts the proton from other compounds and itself makes a conjugate acid categorized as a base. So, when H2CO3 reacts with the water molecule, it donates the proton to the water molecule and itself makes a conjugate base by releasing one proton.
Therefore, H2CO3 also does not fall into the category of Bronsted-Lowry bases.

What is the Conjugate base of H2CO3?
When one proton loses from acid then a compound is formed which is called the conjugate base of that acid, similarly when one proton is added to the base then a compound is formed which is called the conjugate acid of that base.
In technical terms, Compounds differentiated from each other by a single proton(H+) are said to be Conjugate acid-base pairs.
The concept of conjugate acid-base pair.
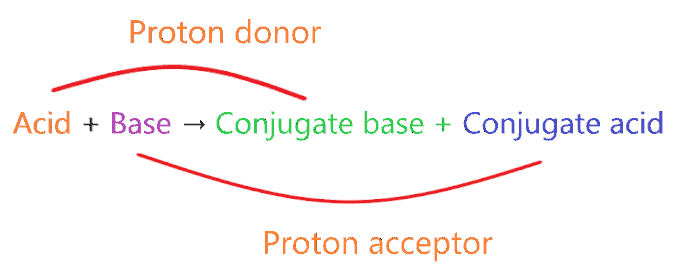
- A very strong acid always forms a weak conjugate base.
- A very strong base always forms a weak conjugate acid.
- A very weak acid always forms a strong conjugate base.
- A very weak base always forms a strong conjugate acid.
So, H2CO3 is a weak acid that forms a conjugate base according to the concept of conjugate acid-base pair.
The conjugate base of H2CO3 is a bicarbonate ion (HCO3–).
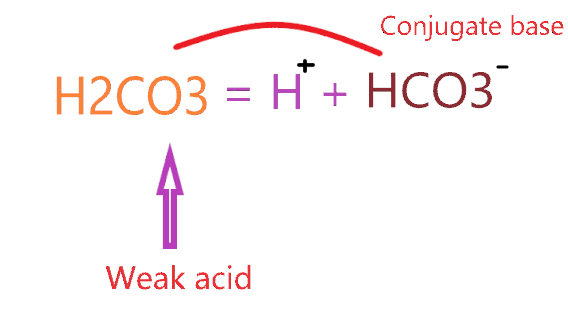
Uses of Carbonic acid
- Carbonic acid is used for the preparation of carbonated water and aerated drinks.
- It is used in various industries such as pharmaceuticals, cosmetics, aesthetics, etc.
- It is used to transport carbon dioxide in the blood.
- It is also used in the production of soft drinks.
Summary
- Is H2CO3 an acid or base? H2CO3 is an acid. Because on dissociation in aqueous solution, it liberates some H+ ion, which shows its acidic nature.
- H2CO3 is acting as an Arrhenius acid and Bronsted-Lowry acid.
- The conjugate base of Carbonic acid (H2CO3) is a bicarbonate ion (HCO3–).
- H2CO3 is a weak acid. Because it contains fewer hydrogen ions in its aqueous solution as it only dissociates partially in the water.
- The Ka value for carbonic acid is 4.2 × 10-7 which is way lower than recommended value for a strong acid (Ka > 1). hence, H2CO3 is a weak acid.
- Carbonic acid is a dibasic acid because when one mole of it is dissolved in water, it furnishes two hydrogen ions.
About the author
Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. The team at Topblogtenz includes experts like experienced researchers, professors, and educators, with the goal of making complex subjects like chemistry accessible and understandable for all. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/

