Is Hydrogen cyanide (HCN) an acid or base? - Strong or Weak

Hydrogen cyanide is one of the most toxic chemical compounds in chemistry, contact with it almost instantly kills you by stopping the oxygenation of critical tissue. It has the chemical formula of HCN. Hydrogen cyanide is a colorless gas and has a strong pungent odor that causes irritation in the eyes and some other respiratory issues.
In this article, we will discuss Is Hydrogen cyanide(HCN) an acid or base? Its nature (Strong or weak), etc. Just hold your eyes on the screen!
So, Is HCN an acid or base? HCN is an acid, with a pKa of 9.2. When hydrogen cyanide is mixed in an aqueous solution, it gives away one proton(H+) and one CN–. The solution containing one hydrogen and one cyanide anion(CN–) is called hydrocyanic acid.
| Name of Molecule | Hydrogen cyanide |
| Chemical formula | HCN |
| Molar mass | 27.025 g/mol |
| Nature | Weak acid |
| Conjugate base | CN– |
| Conjugate acid | HCNH+ |
| Acidity (pKa) | 9.21 |
Why HCN is an acid and not a base?
Do you know? you can easily guess up by looking at the compound whether it is acid or base. Let’s see how.
Most of the acidic compound starts with hydrogen-like H2SO4, HCl, HBr, HNO3, H2CO3 and many more. And most of the basic compounds end with hydroxide groups (OH) like NaOH, KOH, LiOH, etc.
All these acidic compounds, when dissolved in water breaks apart into hydrogen ion and a basic compound breaks apart into OH– ion. Most acid-base theories in chemistry state that acid donates H+ ions and bases donate OH- ion.
In the case of HCN, when dissolved in water it furnishes H+ and CN– ions in solution. The presence of an H+ ion in an aqueous solution makes the HCN nature acidic.
Now check if HCN is an acid or base, with the two important acid-base theories we have (a). Arrhenius theory (b). Bronsted-Lowry theory
(a). Arrhenius theory
This theory said that a substance behaves as an acid when it is ready to give off the hydrogen ion on dissolving in an aqueous solution. And a substance behaves as a base when it releases OH– ion on dissolving in an aqueous solution.
Or a substance is said to be acid when it increases the concentration of H+ ion in solution and a substance is said to be base when it increases the concentration of OH– in solution.
Now check HCN nature as per Arrhenius theory-
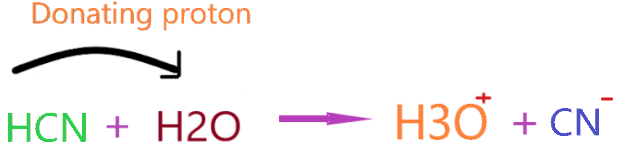
As you see in the above reaction, hydrogen cyanide releases proton on dissolving in an aqueous solution, and, therefore increases the hydrogen ion concentration in the final solution.
Simply, you can check the number of hydrogen ions before and after in solution to verify Arrhenius acid theory.
In the above reaction, we have only two hydrogens on the left side but after reacting with HCN, these hydrogens turn into three. Definitely, there is some increment in hydrogen ions.
So, we can proudly say HCN is an Arrhenius acid without any doubt!
(b). Bronsted-Lowry theory
Here comes another acid-base theory that states a substance is said to be acid when it donates the proton to other species and makes a conjugate base by losing one proton from itself.
And for the base, it states that a substance is said to be base when it accepts the donated proton and makes a conjugate acid by adding one proton to itself.
Let’s consider the reaction of HCN with H2O.
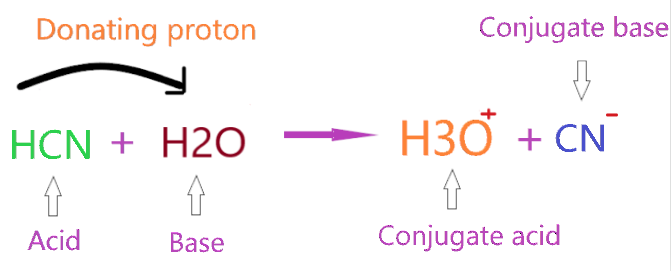
As you see in the above figure, HCN donates one proton to the H2O compound and forms a conjugate base(CN–) by losing one proton from itself, and H2O accepts this donated proton and makes a conjugate acid (H3O+) by adding one proton to itself.
So, in the above reaction, HCN act as a Bronsted-Lowry acid since it donates the proton, and H2O acts as a Bronsted-Lowry base since it accepts the proton.
Is Hydrocyanic (HCN) a strong or weak acid?
The concept of a strong and weak acid is very important to distinguish between the strength of any acid.
A strong acid is referred to as those acids which ionize 100% on dissolving in a solution means all moles of the compound dissociate completely into ions and liberates a large number of H+ in the final solution. Examples of strong acids:- HCl, HNO3, H2SO4, HBr, etc.
Also, Read:-
- Is H2SO4 a strong acid?
- Is HNO3 a strong acid?
- Is HCl a strong acid?
- Is HI a strong acid?
- Is HClO4 a strong acid?
- Is HBr a strong acid?
A weak acid is referred to as those acids which do not ionize 100% on dissolving in a solution means not all moles of the compound dissociate into ions, some molecules left undissociated in the solution. Examples of weak acids:- H3PO4, CH3COOH, NH4+, HF, HNO2, etc.
Also, Read:-
- Is CH3COOH a weak acid?
- Is HF a weak acid?
- Is HNO2 a weak acid?
- Is HCOOH a weak acid?
- Is H3PO4 a weak acid?
- Is H2CO3 a weak acid?
- Is NH4+ a weak acid?
List of some important acid and base-

Now, Is HCN a strong or weak acid? HCN is a weak acid. Since it doesn’t ionize completely to yield H+ ions on dissolving in an aqueous solution. It means, that when HCN is dissolved in an aqueous solution, some parts of it remain undissociated in the solution.
Hence, the number of hydrogen ions in the final solution has a lower amount due to only partial dissociation of HCN.
Now the question arises, why HCN partially dissociates in solution and acquires the weak acid strength. For understanding this, we have to look at some factors that affect the strength of acidic compounds.
We only consider two important factors that can influence the nature of the compound. (1). Polarity (2). Electronegativity
These two factors correlated to each other, so for understanding these factors in a better way, we jotted some points that cover the concept of these factors used in determining the strength of the compound.
- The larger the electronegativity difference between the atoms, the more the bond becomes polar in between them.
- A polar bond can easily break in water solution, hence deprotonation from polar molecules becomes easy as compared to a non-polar molecule.
- More the polar nature of the molecule, high is the acidic strength as it is easier for the proton to leave the molecule.
- If the hydrogen atom is attached to the more electronegative atom, then it is more acidic.
Now considers the above factor to determine why HCN act as weak acid?
The electronegativity of carbon is 2.55, for hydrogen, it is 2.2, and for nitrogen, its value is 3.04. In an HCN molecule, hydrogen is connected to carbon with the help of a single bond, and carbon is attached to nitrogen with the help of three bonds.
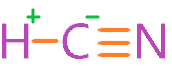
As we know, the easy removal of a proton from a molecule is directly proportional to high acidic strength.
In an HCN molecule, hydrogen is attached to carbon and their electronegativity difference is 0.35, as per the Pauling scale, 0.4 to 1.7 electronegativity differences are required to qualify for a polar covalent bond.
But, 0.35 electronegativity differences in somewhat near to 0.4, so we can consider a weak polar bond in H-C.
Also, the individual electronegativity of hydrogen and carbon is near to each other, hence the charges distribution between them is somewhat uniform.
Therefore, the strength of the bond between hydrogen and carbon is strong as it requires more energy to break the bond, this makes the breaking of hydrogen atoms difficult when solute in water.
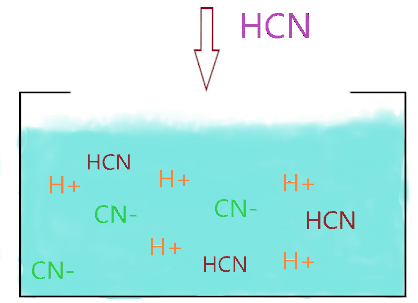 As you see in the above figure when an HCN molecule is dissolved in an aqueous solution, it starts to split into H+ and CN– ions, and due to the weakly acidic nature of HCN, it only dissociates partially into ions and some HCN molecules don’t break at all and present between the splitting ions in solution.
As you see in the above figure when an HCN molecule is dissolved in an aqueous solution, it starts to split into H+ and CN– ions, and due to the weakly acidic nature of HCN, it only dissociates partially into ions and some HCN molecules don’t break at all and present between the splitting ions in solution.
Also, the dissociation constant(Ka) value for HCN is 6.2 × 10-10, which’s really low for qualifying as a strong acid.
⇒ Ka >> 1 (Strong acid)
⇒ Ka << 1 (Weak acid)
I hope you strongly capture the concept of the HCN acidic strength.
Also Read:
What is the conjugate base of HCN acid?
Conjugate acid is an acid that is formed when the parent base compound gains one proton and the conjugate base is a base that is formed when the parent acid compound loses one proton.
As per Bronsted-Lowry theory, HCN is an acid that donates one proton to a water molecule and forms a base (CN–) known as the conjugate base of an acid(HCN).
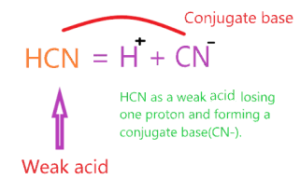
∴ The conjugate base of HCN is CN–.
Uses of Hydrocyanic acid
- Hydrocyanic acid is used as a horticultural fumigant.
- It is used to kill rodents found in grain house bins, warehouses, greenhouses, etc.
- It is used in many chemical compounds as a precursor.
- It is used in the production of synthetic fiber, plastics, dyes, and pesticides.
Properties of Hydrocyanic acid
- It is an extremely poisonous and flammable liquid.
- It is miscible in water and ethanol.
- It has a boiling point of 26 °C and a melting point of −13.29 °C.
- It has an odor-like bitter almond smell.
Summary
Hydrogen cyanide also called prussic acid is one of the dangerous compounds and highly poisonous. It is a linear molecule with a triple bond between carbon and nitrogen. We covered the important detail of the acidity nature of HCN in this article.
- The pKa value of HCN is 9.2.
- Is Hydrocyanic (HCN) an acid or base? HCN is an acid. It has a proton to lose when dissolved in an aqueous solution.
- HCN is acting as an Arrhenius acid and Bronsted-Lowry acid.
- The conjugate base of HCN is Cyanide(CN–).
- However, HCN is a weak acid, due to only partial dissociation of its ions in an aqueous solution, also the dissociation constant (Ka) for HCN is 6.2 × 10-10, which is considered far low for the strong acid.
About the author
Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. The team at Topblogtenz includes experts like experienced researchers, professors, and educators, with the goal of making complex subjects like chemistry accessible and understandable for all. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/

