Is NaHCO3 (Baking soda) an acid or base? - Sodium bicarbonate

Sodium carbonate is made up of sodium cation and bicarbonate anion having a chemical formula NaHCO3. It is also known as baking soda or bicarbonate soda. It appears as an odorless white crystalline powder. It has a slightly salty or bitter taste and is most commonly used as a pH buffering agent.
In this article, we will discuss Is NaHCO3 an acid or base? Is it acidic or basic salt? and its other important properties with all possible explanations.
So, Is NaHCO3 (Baking soda) an acid or base? Baking soda (NaHCO3) is amphoteric, which means it can act as acid as well as the base. The pH value of baking soda is around 8, which shows, it is mildly alkaline or base in nature, so when baking soda is dissolved in water, it produces a basic solution.
In short, Baking soda or Sodium bicarbonate (NaHCO3) can either act as an acid as well base, but its aqueous solution is slightly alkaline in nature.
Therefore, the alkaline solution of baking soda is used to neutralize the level of acid.
| Name of Molecule | Sodium bicarbonate or Baking soda |
| Chemical formula | NaHCO3 |
| Nature | Acid as well as Base |
| pH | 8 – 9 |
| Acidity (pKa) | 10.329 |
How does NaHCO3 (Baking soda) act as acid as well as a base?

As we know, Acid has a pH level from 1 to 6 and the base has a pH level from 8 to 14. Sodium bicarbonate has a pH level of around 8-9 which means it is in the range of basic on the pH scale. So, it can be said that Baking soda (NaHCO3) is a mildly alkaline substance
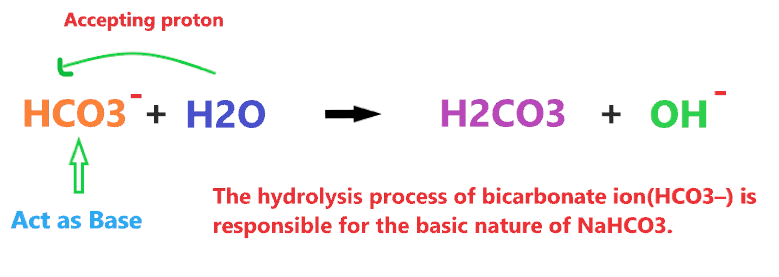
But, How NaHCO3 can act as a base? When NaHCO3 is dissolved in water, it dissociates into two ions Na+ and HCO3–. The Na+ ion is just a spectator ion as it is a cation of the strong base(NaOH), hence, it doesn’t take part in the chemical reaction whereas the HCO3– ion is the anion of the weak acid that can easily undergo hydrolysis.
The hydrolysis process of bicarbonate ion(HCO3–) is responsible for the basic nature of NaHCO3.
As per Bronsted-Lowry base theory, a compound is said to base when it accepts the proton from reacting species.
And according to the Arrhenius base theory, a compound is said to be base when it produce OH– ions in water solution.
So, the anion(HCO3–) of sodium bicarbonate(NaHCO3), when dissolved in water, accepts the proton from the water molecule and produces OH– ions. Hence, according to the above definition, HCO3– act as an Arrhenius base as well Bronsted Lowry base when reacting with the water molecules.
So, this is how NaHCO3 acts as a base because of the hydrolysis of its bicarbonate ion(HCO3–).
Now, How does NaHCO3 act as an acid? When its bicarbonate ion(HCO3–) reacts with a strong base such as OH-, it will act as an acid. This is because a stronger base has more ability to “accept the proton”.
So, when HCO3– reacts with a compound having OH– ion, then HCO3– will act as an acid and therefore, have to donate a proton. And anything that donates the proton is considered as acid as per Bronsted-Lowry theory.

Bronsted-Lowry’s theory for acid said that “the acid is the substance that donates the proton to reacting species”.
As we see in the above figure, HCO3– is donating the proton to OH– and forming H2O and carbonate ion(CO32-).
So, here, HCO3– acts as an acid in presence of strong bases.
∴ That’s all, Sodium bicarbonate or Baking soda (NaHCO3) acts as an acid as well as a base because of its bicarbonate anion(HCO3–) amphoteric activity.
⇒ HCO3– + H2O → H2CO3 + OH– [∴ HCO3– act as base]
⇒ HCO3– + OH– → CO32- + H2O [∴ HCO3– act as acid]
Also Read:-
Is Baking soda (NaHCO3) acidic salt or basic salt?
Have you ever heard what these terms mean by “Acidic salt”, or “Basic salt”?
Acidic salt: When a neutralization reaction carries out between a weak base and strong acid then the salt is formed which is called acidic salt. Examples of acidic salt – NH4Cl, NH4NO3, NH4Br, etc.
Also Read:-
Basic salt: According to the concept of salt, when a neutralization reaction carries out between a strong base and weak acid then the salt is formed which is called a basic salt. Examples of basic salt – are NaCN, Soap, Na2CO3, etc.
Also Read:-
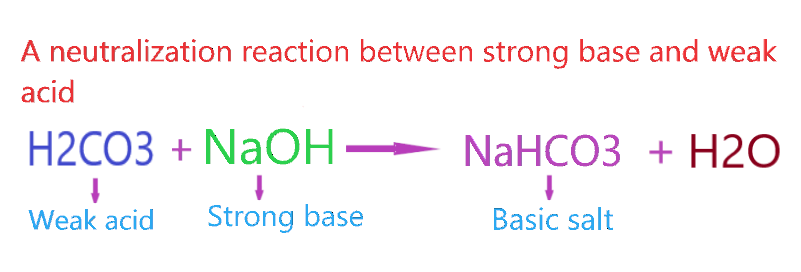
Ok, Now come to the point Is NaHCO3 act as basic salt? As we know, Sodium bicarbonate is formed when NaOH(Strong base) reacts with H2CO3(Weak acid). They react with each other in the molar ratio of 1:1.
⇒ NaOH + H2CO3 → NaHCO3 + H2O
As per the concepts of salts, a combination of a strong base and weak acid leads to the formation of a basic salt.
The nature of salt(acidic or basic) is dependent on the strength of the component in the acid-base reaction.
- An aqueous solution of stronger acid and weak base will acquire more property of acidic instead of a base.
- An aqueous solution of a stronger base and weak acid will acquire more property of basic instead of an acid.
An acidic salt is a salt that has at least 1 replaceable H+ ion.
So, NaHCO3 can also behave as acidic salt because it has still one replaceable H+ ion when reacts with the bases.
⇒ NaHCO3+ NaOH → H2O + Na2CO3
When sodium bicarbonate reacts with a strong base, it undergoes complete neutralization and forms the water and sodium carbonate. Therefore, in this case, it acts as acidic salt due to the presence of one replaceable H+ ion.
At last, we can say Baking soda (NaHCO3) is basic salt because it is formed from the neutralization of a strong base(NaOH) with a weak acid(H2CO3) and it can act as acidic salt also due to the presence of an ionizable hydrogen ion in it.
Why the aqueous solution of NaHCO3 becomes basic?
An aqueous solution having a pH value of more than 7 is called a basic aqueous solution.
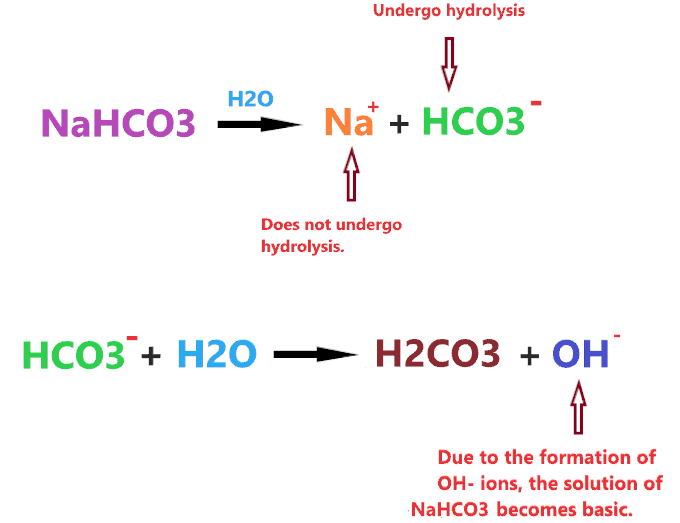
When sodium carbonate is dissolved in water, it dissociates into two ions Na+ and HCO3–.
⇒ NaHCO3 → Na+ + HCO3–
The Na+ is a cation of strong base (NaOH), hence, it will not undergo hydrolysis in water and doesn’t affect the pH of the solution. But the HCO3– is the anion of a weak acid that gets hydrolysis in water to form carbonic acid(H2CO3), leaving free OH– ions that make the solution basic.
⇒ HCO3– + H2O → H2CO3 + OH–
The weak acid like H2CO3 remains mostly unionized in the solution, hence, the final solution contains more OH– ion than H+ ion.
Therefore, with the presence of additional OH– ions in the aqueous solution, the Baking soda (NaHCO3) solution becomes basic in nature.
Note: An aqueous solution nature depends on the H+ and OH– ions. When more OH– ions are present in an aqueous solution, then the solution becomes basic, and when more H+ ions are present in an aqueous solution, the solution becomes acidic.
Also, when the number of H+ and OH– ions same in an aqueous solution, then the solution is said to be neutral.
Also check:-
Why NaHCO3 is used to neutralize an acid?
As we know, sodium bicarbonate is basic salt in nature, and its pH value that lies between 8 – 9. So, when NaHCO3 is added to a solution of strong acid, it will form sodium chloride salt and carbonic acid.
⇒ NaHCO3 + HCl → H2CO3 + NaCl
Carbonic acid is dibasic acid that has the chemical formula of H2CO3. It is unstable acid that means it can easily decompose into water and carbon dioxide.
So, when NaHCO3 is added to a solution of strong acid, carbon dioxide is emitted, left the solution completely neutral.
⇒ NaHCO3 + HCl → NaCl + H2O + CO2(g)
Sodium bicarbonate doesn’t generate any type of toxic gas that’s why it is used as an ingredient of stomach acid neutralizer.
At last, sodium bicarbonate is a salt that split into two ions (Na+ + HCO3–) on dissolving in water and the hydrolysis of bicarbonate ion makes its solution alkaline which is further used for neutralizing the acid.
Uses of Baking soda
- Baking soda is used as an antacid to neutralize the stomach acid that helps in reducing ulcer pain and heartburn.
- It is used for lowering the pain of minor skin injury by neutralizing the toxins found on the skin surface.
- It is used to extinguish small intensity fires by being thrown over the fire.
- Baking soda is used to control the odor. Generally, it is used to absorb the bad odor in the refrigerator by placing the open box that contains baking soda.
- It is used to remove paint and to reduce corrosion.
- Baking soda has anticaries and abrasive properties so, it is used as an ingredient in some mouthwashes that helps to neutralize the production of acid in the mouth.
- Baking soda can be mixed with water to use on the body or as a facial scrub for removing dirt and bacteria.
- NaHCO3 is used to regulate the pH of a substance with the help of its amphoteric nature.
- When baking soda is dissolved in water, it will make some carboxylic acid which can be used as a cleansing agent.
- Baking soda is used for cleaning the floor, oven, microwave, furniture, and other essential household items.
Major use of baking soda: In cooking, baking soda is primarily used in baking as a leavening agent. When it reacts with acid, carbon dioxide is released, which causes expansion of the batter and forms the characteristic texture and grain in cakes, quick breads, soda bread, and other baked and fried foods.
Summary
Sodium bicarbonate is a white solid crystalline chemical compound usually in powder form. It is also called baking soda or bicarbonate soda having a pH value of around 8. Baking soda is most commonly used in cooking as a leavening agent. At last, we will take an overview of this article on whether Baking soda (NaHCO3) is an acid or base?
- Is NaHCO3 (Baking soda) acid or base? Baking soda is a base, with a pH level of around 8, its aqueous solution is slightly basic. But Baking soda (NaHCO3) can act as acid as well as a base, Because of its bicarbonate anion (HCO3–) amphoteric activity.
- Baking soda (NaHCO3) is basic salt. It is formed from the neutralization of a strong base, namely Sodium hydroxide (NaOH), and a weak acid, namely Carbonic acid (H2CO3). It can act as acidic salt also due to the presence of an ionizable hydrogen ion in it.
- Baking soda (NaHCO3) aqueous solution is alkaline or basic. Because of the presence of additional OH– ions produced from the hydrolysis of its bicarbonate ion(HCO3–).
About the author
Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. The team at Topblogtenz includes experts like experienced researchers, professors, and educators, with the goal of making complex subjects like chemistry accessible and understandable for all. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/

