Is H3PO4 an acid or base? Weak vs Strong - Conjugate base

Phosphoric acid is colorless, odorless appears as a clear liquid or transparent crystalline solid with the chemical formula H3PO4. It is also known as orthophosphoric acid or phosphoric(V) acid. It is corrosive to metal and tissue.
It can also severely irritate the skin and damage the eyes. In this article, we will learn Is phosphoric (H3PO4) an acid or base? Its conjugates pair, nature of strength(weak or strong), etc.
So, Is H3PO4 an acid or base? H3PO4 is a weak acid. Because it undergoes partial dissociation on dissolving in water or aqueous solution and produces a low amount of hydrogen ion. Lower the hydrogen ion in the solution, less is the strength of acidity of the compound.
The pKa value of H3PO4 is around 2.14. “The lower the pKa, the stronger the acid and the greater the ability to donate a proton in aqueous solution”.
The pKa value of a strong acid is below zero.
| Name of Molecule | Phosphoric acid |
| Chemical formula | H3PO4 |
| Molar mass | 97.994 g·mol−1 |
| Conjugate base | H2PO4– |
| Nature | Weak acid |
| Acidity (pKa) | 2.14 |
Why H3PO4 acts as an acid?
H3PO4 is considered an acid. It releases H+ ions when dissolved in an aqueous solution. And acid is a substance that donates the proton to other compounds or releases H+ ions in a water solution. Hence, H3PO4 is acid as it releases H+ ions in a water solution.
⇒ H3PO4 ⇌ H+ + H2PO4−
Let’s understand whether H3PO4 acid or not with the help of two important theories. (a). Arrhenius theory (b). Bronsted-Lowry theory
1. Arrhenius’s theory for acid:
According to Arrhenius’s theory for acid, an acid is a substance that produces H+ ions when dissolved in an aqueous solution. So, in the case of H3PO4, it breaks into two ions (H+ and H2PO4–) when dissolved in an aqueous solution.
Hence, H3PO4 acts as acid as per Arrhenius’s theory.
2. Bronsted-Lowry theory for acid:
According to this theory, an acid substance will donate the proton to another species and form the conjugate base. So, in the case of H3PO4, when it reacted with water, it donates the proton to the H2O and itself makes the H2PO4– conjugate base by losing one proton.
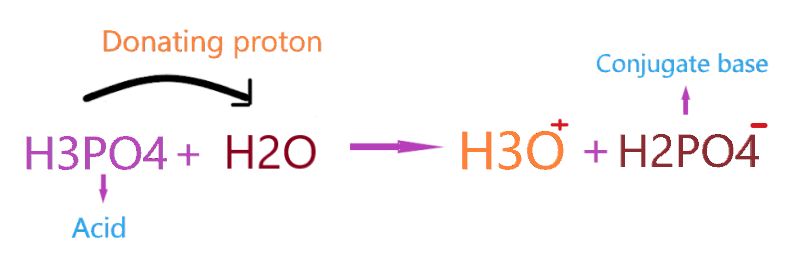
As you see in the above reaction, H3PO4 loses the one proton and donates it to H2O making hydronium ion(H3O+) by gaining one proton and H3PO4 makes conjugate base H2PO4– by losing one proton.
So, in the above reaction, H3PO4 acts as a Bronsted-Lowry acid, and H2O acts as a Bronsted-Lowry base.
The main advantage of the Bronsted-Lowry theory is that it is not restricted to neutral molecules only, it covers the ionic species as well. This theory also explains the behavior of acid base in an aqueous or non-aqueous medium.
Is Phosphoric acid (H3PO4) strong or weak?
To understand whether H3PO4 is a strong or weak acid, first understand the basic difference between a strong acid and weak acid.
A strong acid is a compound that is 100% ionized or completely dissociates in an aqueous solution and gives a high amount of hydrogen ions. They have a low pH value and good electrical conductivity properties. Example: HCl, HNO3, H2SO4, HBr, HClO4, etc.
Also, Read:-
- Is H2SO4 a strong acid?
- Is HNO3 a strong acid?
- Is HCl a strong acid?
- Is HI a strong acid?
- Is HClO4 a strong acid?
- Is HBr a strong acid?
And weak acid is a compound that partially dissociates or not 100% ionized in an aqueous solution and gives a low amount of hydrogen ions. They have a high pKa or pH value with moderate electrical conductivity property. Example – CH3COOH, HCN, NH4+, HF, H2CO3, etc.
Also, Read:-
- Is CH3COOH a weak acid?
- Is HF a weak acid?
- Is HCN a weak acid?
- Is HNO2 a weak acid?
- Is HCOOH a weak acid?
- Is H2CO3 a weak acid?
- Is NH4+ a weak acid?
Now, Is Phosphoric strong or weak acid? H3PO4 is a weak acid. Because it doesn’t dissociate completely in an aqueous solution to give H+ ions. It means, that, when H3PO4 is dissolved in an aqueous solution then some moles of it remain undissociated in the solution and are not fully ionized to yield H+ ions.
⇒ H3PO4 + H2O ⇔ H3O+ + H2PO4− [pKa = 2.14]
⇒ H2PO4−+ H2O ⇔ H3O+ + HPO42− [pKa = 7.20]
⇒ HPO42−+ H2O ⇔ H3O+ + PO43− [pKa = 12.37]
The acid dissociation constant(K1) for the first reaction is 7.25 × 10-3, for the second reaction(K2), it is 6.31 × 10-8 and for the third reaction(K3), it is 3.98 × 10-13.
∴ K1 > K2 > K3 [More the acid dissociation constant value, high is the acidic strength of compound]
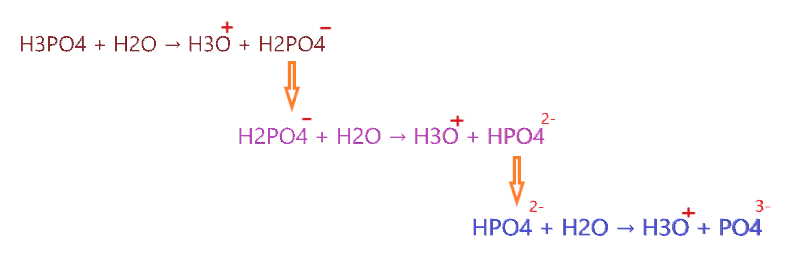
As you see in the above reactions, After the first dissociation, H3PO4 is not fully ionized which means some H+ ions remain bound to it. Phosphoric acid takes three processes to fully dissociate into ions(even then its most of the dissociated ions reform with each other and make H3PO4 again). Hence it is considered a weak acid.
The first process:- In the first process, H3PO4 dissociates into (H2PO4– and H+), but H2PO4– is not much stable, hence it tried to reform with H+ ions and makes H3PO4 again.
Second process: In the second process, H2PO4– breaks into the H+ and HPO42-, even though H2PO4– is not so stable and considered weak acid in nature, it still loses some proton in water solution due to its weakly acidic nature.
Third Process: In this process, HPO42- as a very weak acid release the proton from it and forms PO43-.
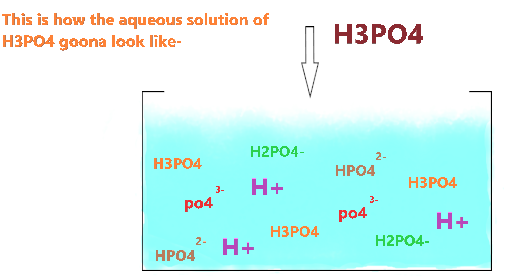
As you see, the aqueous solution of H3PO4 contains ions, whole molecules, few H+ ions, etc because of its weak acidic nature that leads to only partially dissociation of ions in solution.
Most splitting ions regain with H+ and form the original acid molecule(H3PO4 ). Hence, the amount of hydrogen ions becomes low, and all this makes the aqueous solution less acidic.
Note: Removal of one proton from H3PO4 gives dihydrogen phosphate ion(H2PO4−), two protons give hydrogen phosphate(HPO42−) and when all three protons are removed, it gives orthophosphate(PO43−).
Also Read:
Here’s the list of some common acids and bases with their strength.

Why H3PO4 is tribasic acid?
Tribasic acid is also known as triprotic acid which produced three hydroniums or hydrogen(H+) ions for each molecule of acid ionized. A tribasic acid contains three replaceable hydrogen ions.
So, H3PO4 is tribasic acid because it contains three hydrogen ion that is replaceable when acid is ionized or dissociated in an aqueous solution.
⇒ H3PO4 → 3H+ + PO43-
What is the basicity of phosphoric acid?
The basicity of acid defined as the number of hydrogen ions or H+ that can be produced when one molecule of acid is ionized or dissociated in an aqueous solution. In the case of phosphoric acid, three hydrogen ions are produced when ionized in an aqueous solution.
Therefore, the basicity of H3PO4 is three.
What is the Conjugate base of H3PO4?
When one proton loses from acid then a compound is formed which is called the conjugate base of that acid, similarly when one proton is added to the base then a compound is formed which is called conjugate acid of that base.
The concept of conjugate acid-base pair.
- A very strong acid always forms a weak conjugate base.
- A very strong base always forms a weak conjugate acid.
- A very weak acid always forms a strong conjugate base.
- A very weak base always forms a strong conjugate acid.
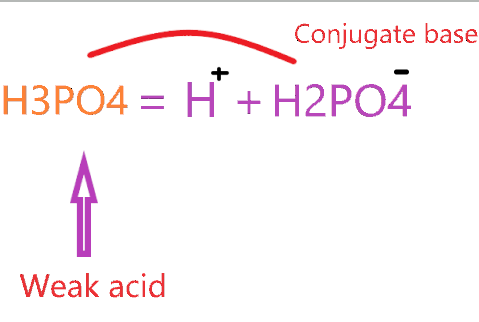
So, H3PO4 is a weak acid that forms a conjugate base by losing one proton from it according to the concept of conjugate acid-base pair.
The conjugate base of H3PO4 is a dihydrogen phosphate anion (H2PO4-).
Why is H3PO4 weaker acid than H3PO3?
H3PO3 is more acidic than H3PO4 because it loses the proton or donates the H+ ion in water easily. H3PO3 is more polar, hence its O-H bond is weaker that causing the H+ ion easier to come off.
Another way to find the acidity strength of oxyacids is by knowing the ratio of double-bonded oxygen and the number of hydrogen ions. “Acidic strength of oxyacids is directly proportional to double-bonded oxygen and inversely proportional to the number of hydrogen ions.
So, in the H3PO4 molecule, there is one oxygen bonded atom and 3 OH– group, the ratio is 1/3 whereas in the H3PO3 molecule, there is one double-bonded atom and 2 OH- group, so the ratio is 1/2.
∴ (1/2) H3PO3 > (1/3) H3PO4
Hence, H3PO3 is more acidic than H3PO4
Also, the pKa value of H3PO3 is lower than H3PO4 which makes H3PO3 more acidic than H3PO4 because the lower the pKa value of the compound, the higher is the acidic strength of that compound.
Acidic properties of Phosphoric acid
All three hydrogens are acidic, with dissociation constants pKa1 = 2.14, pKa2 = 7.20, and pKa3 = 12.37. It follows that, in water solutions, phosphoric acid is mostly dissociated into some combination of its three anions, except at very low pH. The equilibrium equations are:
H3PO4 + H2O ⇌ H3O+ + H2PO4− Ka1= 7.25×10−3 [pKa1 = 2.14]
H2PO4−+ H2O ⇌ H3O+ + HPO42− Ka2= 6.31×10−8 [pKa2 = 7.20]
HPO42−+ H2O ⇌ H3O+ + PO43− Ka3= 3.98×10−13 [pKa3 = 12.37] [Source: wikipedia]
Uses of Phosphoric acid
- It is used in the production of phosphate fertilizer.
- It is used as a dispersing agent, sanitizing agent, etching agent, electrolyte, etc.
- It is used in the pharma industry as teeth whiteners or mouth washing liquids.
- It is used as an additive and flavoring agent in animal or poultry feed.
- Phosphoric acid is used for removing rust from various metals like steel, iron, etc.
- It is used to control the pH level of products or used as a pH modifier.
- Phosphoric acid is used in the production of various products like bath products, hair products, skincare products, make-up, etc.
- It is used in the manufacturing of activated carbon.
Conclusion
Phosphoric acid is also known as orthophosphoric acid or phosphoric(V) acid contains one atom of phosphorus, four atoms of oxygen, and three atoms of hydrogen. It is tribasic in nature as it contains three replaceable hydrogen ion that makes phosphate with three possible sequential deprotonation steps.
- Is H3PO4 an acid or base? H3PO4 is acid. It has a proton or hydrogen ion to donate in an aqueous solution.
- H3PO4 is acting as an Arrhenius acid and Bronsted-Lowry acid.
- The conjugate base of Phosphoric acid (H3PO4) is H2PO4– (dihydrogen phosphate anion).
- Is phosphoric acid strong or weak? H3PO4 is a weak acid. Because some parts of the H+ ion remain bounded when dissociating it in an aqueous solution. hence, it is not 100% ionizable which makes, it a weak acid in nature. It takes three dissociation steps to release all H+ ions in an aqueous solution even then its split ions reform with each other and makes H3PO4 again, hence, H3PO4 never fully dissociates in an aqueous solution which makes it a weak acid.
- The basicity of H3PO3 is 3.
- The acidity (pKa) value of phosphoric acid is 2.14.
About the author
Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. The team at Topblogtenz includes experts like experienced researchers, professors, and educators, with the goal of making complex subjects like chemistry accessible and understandable for all. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/

